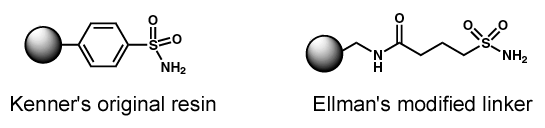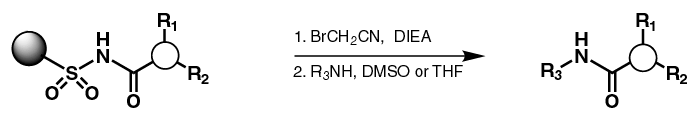|
Rapid
Parallel Synthesis Utilizing Microwave
Irradiation
Brian M.
Glass, Andrew P. Combs*
DuPont Pharmaceuticals
Company, Chemical and Physical Sciences Department,
Experimental
Station, P.O. Box 80500, Wilmington, DE 19880-0500, USA.
Email: [email protected]
Received: 15 August 2001 / Uploaded 22 August 2001
Abstract: Many solid
phase reactions require high temperatures and/or long reaction times to
drive the reactions to completion. Microwave irradiation provides a
useful, expedient, and inexpensive solution to facilitate the rapid
parallel synthesis of large combinatorial libraries. Here we utilize
microwave irradiation and the safety-catch linker to rapidly produce large
libraries of diverse amide and urea products.
Safety-Catch Linker Background
- Initially developed
by Kenner1 for peptide synthesis
- Must be activated
prior to nucleophillic displacement from support
- Stable to both
strongly acidic and basic conditions
- Cleaved with
hydroxide or nucleophillic amines to form acid and primary amide
products
- Activation step and
linker adapted by Ellman, et.al.2 to form more
reactive acylsulfonamide linkage
- Cleavage with
sterically hindered amines and non-nucleophillic amines possible
with heating
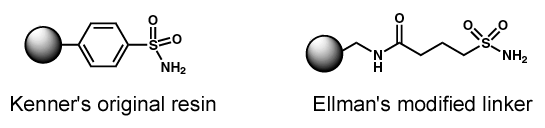
1 Kenner, G.
W.; McDermott, J. R.; Sheppard, R. C. "The Safety Catch Principle in
Solid Phase Peptide Synthesis",Chemical Communications
1971, 636-637.
2 Backes, J.
B.; Virgilio, A. A.; Ellman, J.A. J. Am. Chem. Soc.
1996, 118, 3055-3056. |
Basics of Microwave Chemistry
- Microwave region of
electromagnetic spectrum lies between 1cm and 1m
- Commercial
microwaves operate at 2.45GHz
- Heating effect
primarily due to dielectric polarization
- Molecular rotation
is similar to frequency of radiation and therefore molecule
continually attempts to rotate to align itself with the applied
field and absorb energy
- Larger the
dielectric constant, the greater the coupling with
microwaves
S. Caddick, "Microwave Assisted
Organic Reactions",Tetrahedron 1995, 51,
10403-10432.
Strauss, C. R.; Trainor, R. W.
Aust. J. Chem. 1995, 48,
1665-1692. |
| Safety Catch Strategy
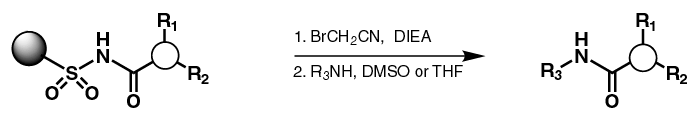
- Scaffold attached to
safety-catch resin
- Resin compatible
with both BOC and FMOC protecting group strategies
- Resin is activated
for cleavage by alkylating the sulfonamide nitrogen
- Compound cleaved
from resin with nucleophillic displacement
- Only libraries of
amide products have been shown in literature. Here we show that
urea products are also possible.
|
| Ureas
Synthesized Using Safety-Catch
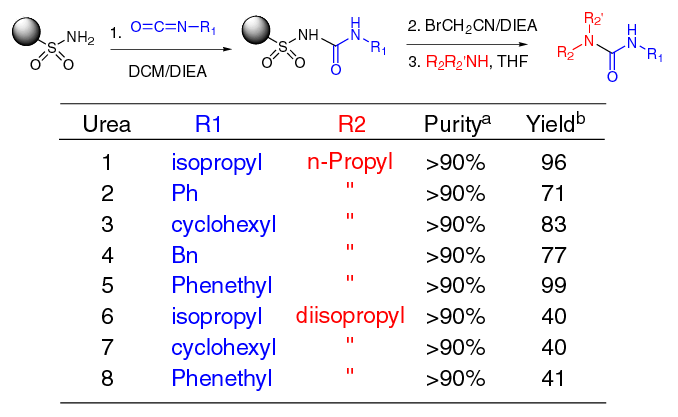
a Purity of
product based on crude 1H NMR. b Purified
yields based on initial loading (0.73 mmol/g) of alkyl safety-catch
linker. |
| Biaryl Ureas on Safety-Catch
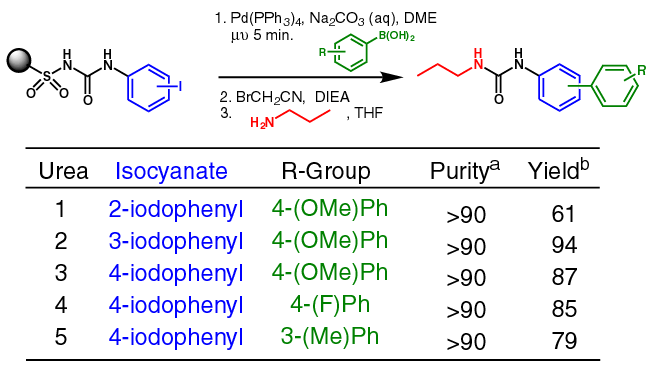
a Purity of
product based on crude 1H NMR. b Purified
yields based on initial loading (0.73 mmol/g) of alkyl safety-catch
linker. |
| Microwave Assisted Library Production

- Scaffold attached to
resin
- Resin activated and
split into filter plates
- Cleavage run in DMSO
solution with limiting amine concentration
- Microwaving drives
reactions with weaker nucleophiles to completion
- Resin washed with
DMSO to bring filtrate to 10mM for assaying (no solvent
evaporation necessary)
88 Diverse
Amines
 |
Rates
of Cleavage for Poor Nucleophiles
 |
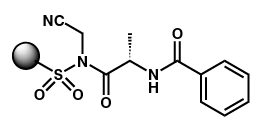
Rates of
cleavage of resin in 0.01M
amine solutions in
DMSO
- Cleavage is
sluggish at room temperature, heating accelerates
rate
- Microwaving
does not further accelerate rate over traditional
heating
- Microwaving to
higher temperatures can drive reactions to completion within
15 minutes
| |
| Microwaving Plates - Temperature Gradients
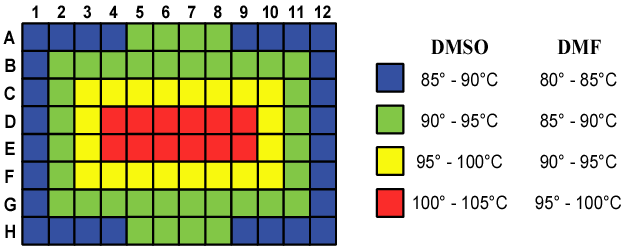
- Continuous
microwaving for 1 minute in commercial oven (1100 watts)
- Polyfiltronics PKP
2mL Filter Plate - holds DMSO solution in plate until vacuum is
applied
- 20 Degree Celsius
Gradient in Plates for DMSO and DMF
|
TLC
Analysis of Library Synthesis
- Convenient way to
rapidly determine library purity
- Sensitive detection
of the presence of leftover amine (ninhydrin stain)
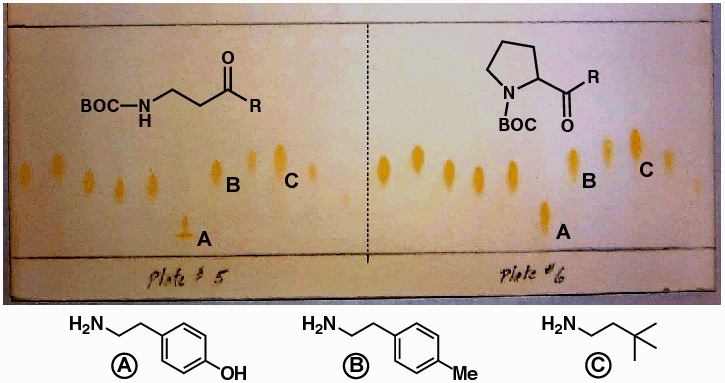
- TLC of identical
amines cleaving 2 different resins shows relative Rf
pattern.
|
Concluding Comments
- 10 plates (880
compounds) synthesized per chemist in two weeks
- Libraries of amides
and ureas can be synthesized in high purity
- >95% Identified
by LCMS
- Proprietary amine
inputs and scaffolds
- Source of large
novel compound libraries for general high throughput
screening
- No expensive
equipment needed
- No special reaction
vessel needed
| |