Physical Chemistry
Similarity Rule and Complementarity Rule
Shu-Kun Lin
Molecular Diversity Preservation International (MDPI)
Sängergasse 25, CH-4054 Basel ([email protected],
http://www.mdpi.org/lin/)
Intermolecualr and intramolecular processes are governed by two rules: Similarity rule (a component in a molecular recognition process loves others of alike properties, such as hydrophobic interaction, p-stacking, similarity in softness of hard-soft-acid-base rules) predicts the affinity of individuals of similar properties. On the contrary, complementarity rule predicts the affinity of individuals of certain different properties. Both types of rule still remain empirical.
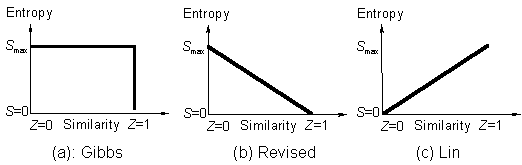
Similarity rule can be explained by similarity principle (Fig. c) [1] after rejection of Gibbs' (Fig. a) and revised (Fig. b) relations of entropy-similarity.

Complementarities during all kinds of donor-acceptor interaction, such as enzyme and substrate combination, which involves hydrogen bond, electrostatic interation and key-and-lock docking, can be defined and quantitatively calculated as parts of reducible information due to the decrease of the numbers of the information recording individual after any successful tight interaction or combination. The resulting structures are more symmetric due to the property offset of the components, which can be explained by symmetry principle (the higher symmetry-higher entropy-higher stability relation), which has been proved by the similarity principle.
1. See http://mdpi.org/lin/similarity/similarity.htm and citations.