[E0034]
THE COMPARATIVE STUDY
OF THE KINETICS OF KNOEVENAGEL CONDENSATION UNDER MICROWAVE AND CONVENTIONAL
CONDITIONS.
Szczepan Bednarz, Dariusz Bogdal*
*e-mail:
[email protected]
Department of Polymer Science and Technology Politechnika Krakowska ul. Warszawska 24, 31-155 Krakow, Poland
Received: 15 August 2001 / Uploaded 22 August 2001
Acceleration of the reaction rates, compared to the normal conditions could
be due to different mechanism of transferring heat, other suggest that
specific nonthermal microwave effect exist. In our investigations, we observed
specific microwave effect.
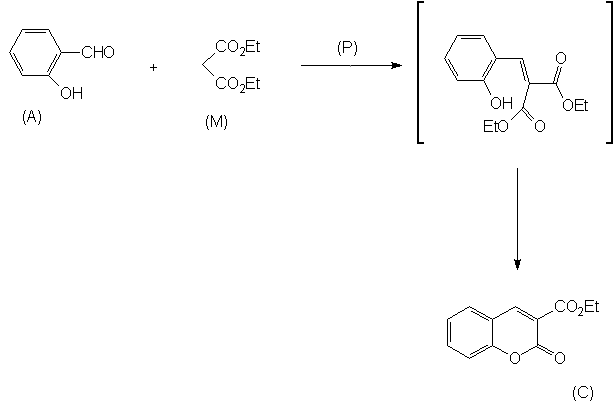
In our work, we studied kinetics
of Knoevenagel condensation [8]
of salicylaldehyde and diethyl malonate, in the presence of piperidine
as a catalyst and toluene as a solvent (Scheme
1). In earlier
work [9]
the rate equation was determined empirically (Scheme
2). We have
measured the reaction rate constant at various temperature under microwave
and conventional conditions (Figure
2, Table
1). The reaction
mixture was analyzed by GCMS and naphtalene was added to the reaction as
an internal standard.
 |
|
|
Knoevenagel condensation reaction rate has been reported to be more than
three times higher during microwave irradiation than conventional heating
[9].
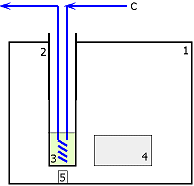
In our work, we studied the influence of microwave power energy on described
chemical systems. We used specific system (Figure
1): a monomode
mirowave reactor (Synthewave 402 - Prolabo), operating at various microwave
powers. It was equipped with an infrared pyrometer to measure reaction
temperatures. Since the reacting mixture strongly absorbs microwave radiation,
we used cyclohexane (minimal microwave absorption) flow in glass cooler
to refrigerate.
 |
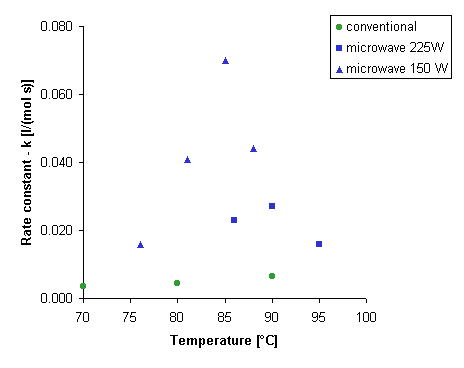
We
observed incomprehensible behaviour of the reaction system (Figure
2). When we
applied 150 W microwave power, reaction rate was higher than with 225 W,
despite of a temperature at the same level. Whereas at 95°C, under
microwave irradiation at 225 W, we observed small rate enhancements. The
influence of the microwave power and the temperature on the kinetics
of the chemical reactions is complex, so if an optimal range of these parameters
exists we can lead chemical processes in maximal rate.
Table
1. Rate
constant for the Knoevenagel condensation of salicylaldehyde, diethyl malonate
in toluene, in the presence of piperidine, under different conditions -
k [l/mol·s].
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
 |
Further study of a similar reaction system and more accurate method of
the determination of the temperature (fiberoptic thermometer) are needed
before a definitive conclusion can be reached.
References
[2] Fini A., Breccia A., Pure Appl. Chem., 1999, 71, 573.
[3] Thostenson E. T., Chou T. W., Composities, 1999, 30, 1055.
[4] Zlotorzynski A., Critic. Rev. Anal. Chem., 1995, 25, 43.
[5] Abramovitch R.A., Huang Z., Chemosphere, 1994, 29,2517.
[6] Varma R.S., Saini K., Tetrahedron Lett., 1998, 39, 1481.
[7] Loupy A., Pigeon P., Ramdani M., Tetrahedron, 1996, 52, 6705.
[8] Bogdal D., J. Chem. Res., (S) 1998, 468.
[9] Bogdal D., monografia PK nr 248, Kraków 1999.