
[A0013]
Stefan Bräse
*, Stefan Dahmen, Matthias LormannRWTH Aachen, Institut für Organische Chemie, Professor-Pirlet-Str. 1, D-52074 Aachen, Germany,
fax: (+49)(0) 241 8888127;email: [email protected]
Received: 30 July 1999 / Uploaded: 10 August 1999
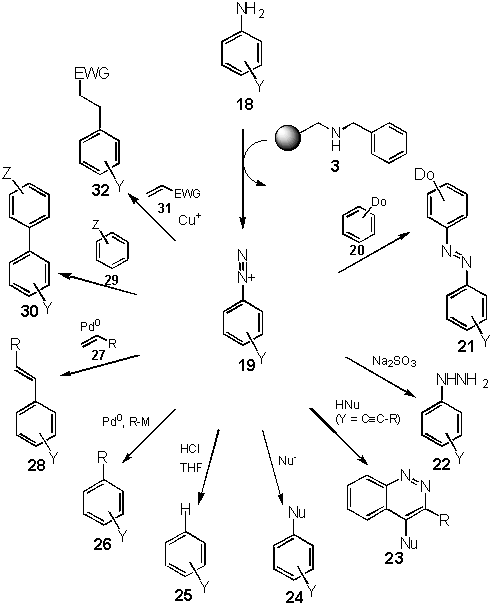
The T1 linker for the attachment of arenes has been described. Various transformations yielded modified arenes. The cleavage from the resin has been conducted to give hydrocarbons ("Traceless linker"), heterocycles (Richter reaction), arylated, alkenylated and alkylated arenes (Heck cleavage protocol), haloarenes, phenols, biaryls and azo compounds. This method is applicable to parallel synthesis and automation.
The solid phase synthesis of organic compounds has gained a renaissance for the last years due to the fact, that operating with resin chemistry can efficiently be used in parallel reactions for high-throughput synthesis.[2] Among the various linker applied for attachment and detachment of organic fragments, multidirectional cleavage,[2] i. e. functionalization by detachment, promised additional diversity which would not bias the libraries produced. Herein, the triazene linker for arenes, called T
1 linker has been described to show the versatility of diazonium type anchoring.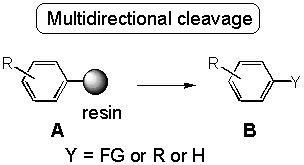
One method for converting arenes into the corresponding functionalized hydrocarbons is the reaction of diazonium compounds. As these compounds react with amines to yield triazenes which can be transformed back into diazonium compounds under mildly acidic conditions, the use of triazenes as linkers is to be a very promising strategy.
Possibilities for T1
The Traceless Linking System: Introduction of a Hydrogen Atom
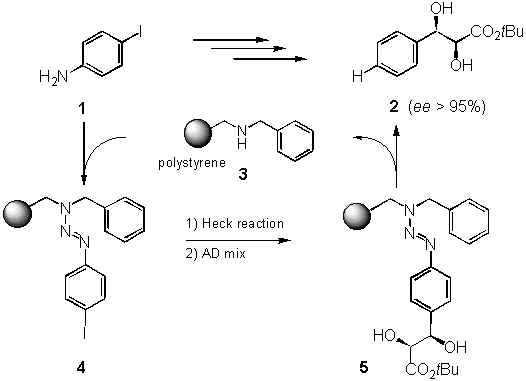
Inspired by the use of triazenes in the total synthesis of vancomycin [3] and the pioneering work of Moore et al. [4] and Tour et al. [5] in the attachment of triazenes to a solid support and the final detachment to give iodoarenes, we were interested in developing a traceless linker system based on triazenes.[6] Starting from a secondary amine resin 3,[7] synthesized from Merrifield resin in one step, and diazonium salts derived from readily available anilines 1, the triazene system 4 was built up in a single step. After chemical transformation, e. g. Heck reaction and asymmetric dihydroxylation (4 ® 5), cleavage can be affected by treatment with acid (e. g. HCl) in the presence of a reducing agent such as THF to give the product 2 in good yield.
Heck-Cross Coupling: Introduction of Carbon Fragments
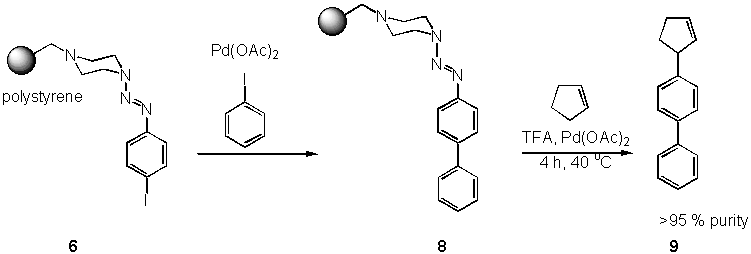
Besides the possibility of conducting traceless cleavage from solid support, a cleavage-cross-coupling can be achieved using palladium-catalysed systems.[8] Diazonium salts are known to undergo insertion of Palladium, thus making them suitable substrates for a Heck reaction.[9] Starting from the triazene resin 6 a reductive palladium-catalyzed biaryl coupling under Heck conditions with iodobenzene (7) gives rise to a resin 8. Liberation of the diazonium salt and subsequent coupling with various alkenes e. g. cyclopentene proceeded smoothly under mild thermal conditions (12 h at 40 oC in MeOH) to give the product 9 in high yield.[10] The use of palladium on charcoal as catalyst allows couplings in lower purity (87%), but is advantageous with regards to the ease of removal by filtration, i.e. the filtrate is nearly colorless and free of palladium compounds. The catalyst can be used for a subsequent hydrogenation reaction thus allowing the formal coupling of alkyl groups.
The Solid Phase Richter reaction: Synthesis of Heterocycles
In the examples above, the diazonium group, upon cleavage from the resin, has been lost by the formation of dinitrogen. However, in liquid phase a suitable nucleophilic ortho-substituent favours cyclization to resulted in heterocyclic structures.[11] This transformation has been known for over a century as Richter (ortho-alkynyl),[12,13] Widman-Stoermer (ortho-alkenyl) [14] or Borsche-Koelsch (ortho-acetyl) [15] reactions to yield cinnoline derivatives, which are interesting building blocks for biologically active compounds. In particular, 4-halocinnolines can serve as valuable starting materials owing the ease of substitution by nitrogen nucleophiles.

Starting from benzylaminomethyl polystyrene 3 [7] (1-2% cross-linked with DVB, 100-200 mesh), the required diverse ortho-haloaryl resins 10 were prepared from substituted ortho-haloanilines in good yields. The palladium-catalyzed cross-coupling under standard conditions [Pd(OAc)2, NEt3, DMF, 80 °C] with different alkynes (trimethylsilylethyne, phenylethyne, 1-heptyne, 1-benzyloxy-1-ethynylcyclopentane) afforded the ortho-alkynylarene resins 11. It should be noted that copper was omitted due to coordination of the triazene moiety hence leading to traces of copper in the final product. Copper derivatives are more toxic than the palladium analogues. The Richter cleavage reactions were conducted with aqueous hydrogen chloride or hydrogen bromide in acetone at room temperature (1 h). Filtration and dilution with water afforded the cinnolines such as 15-17 in yields of 47 to 95% and with 60-95% purity without any further purification (NMR, GC, GC-MS).[16] The cleavage was also successfully conducted in a 2 ´ 11 matrix on the Bohdan MiniBlock.[17]
The Bohdan MiniblockTM
While under more acidic conditions (20 equiv. of acid, 1 molar),
trimethylsilylethyne can serve as an ethyne equivalent due to the lost of the silyl group,
dilute acid favors the formation of silyl derivatives like 15-Br. Hydrogen fluoride
or hydrogen iodide failed to give the desired compounds. Longer reaction times led to
increased formation of 4-hydroxycinnolines by a hydrolysis reaction (e. g. 14-OH, 13-OH,
40-65% yield), which also have been found as major product
when conducting the reaction in a more diluted acidic solution (2 equiv. of acid, 0.1
molar).[18]
Other Multidirectional Cleavage Reactions: Synthesis of Phenols, Arylhalides and Azo Compounds
Reaction of triazene resins with trimethylsilyl halides (I, Br, Cl) leads to the formation of aryl halides (24, Nu = halogen).[19] In the presence of oxygen nucleophiles such as water or alcohols, cleavage by acids yield phenols and aryl ether (24, Nu = OH and OR).[19] Azo compounds 21 are accessible by cleavage and subsequent azo coupling. Biaryls 20 can be synthesized by a Gomberg-Bachmann reaction, Meerwein alkylation products 31 are also accessible. Reduction of the diazonium fragments leads to hydrazines 22.
The T1 linker is distinct from the existing alternatives by its accessibility, robustness and ability to regenerate the resin. The broadness of applications in a number of organic reactions can be anticipated. The T1 triazene linker system is now commercially available).[7]
It is a great pleasure to acknowledge the important contributions of my co-worker on this project. Their names appear in the original publications or in the notes. This work was supported by the Deutsche Forschungsgemeinschaft (BR 1750/2-1) and the Fonds der Chemischen Industrie (Liebig stipend to S. B.). I thank the companies NovaBioChem, Bayer AG, and Grünenthal GmbH for donations of chemicals and financial support, Bohdan Inc. for the opportunity using the MiniBlock synthesizer, Prof. Dieter Enders for continuos interest and support of our work as well as Johannes Köbberling for beneficial discussions and contributions concerning this work.
The polymeric compounds were characterized by IR and elemental analysis; the products obtained after cleavage were characterized by NMR methods (1H NMR, partially 13C NMR) and MS. The purity was controlled by NMR, HPLC, LC-MS, GC and/or GC-MS. Elemental analyses were performed at Mikroanalytisches Labor des Instituts für Organische Chemie der RWTH Aachen (Heraeus CHN-O-Rapid). All solvents have been distilled before use. Merrifield resin (1-2% cross-linked, 0.63 mmol g-1, 200-400 mesh) was obtained from NovaBioChem-CalBioChem. All resins were washed sequentially by using a vacuum reservoir connected to a sintered glass. Cleavage was conducted using Teflon tubes with a frit connected to a vacuum line or with a glass pipette filled with glass wool. Evaporation of the solvent was achieved using rota vapor and/or high vacuum (ca. 0.1 mbar). MS: EI-HRMS: Finnigan MAT 95 (70 eV). GC analytic: Siemens Sichromat 2 und 3, 25 m ´ 0.25 mm capillary column with OV-1-CB (carrier gas N2, column A), SE-54 (carrier gas N2, column B) or Lipodex E (carrier gas H2, column C). The content of the chromatograms refers to uncorrected values in percent and are the integral of the peaks.
Example 1. Two step Heck type alkene coupling and Traceless cleavage
Heck type alkene coupling
In a pressure resistant Pyrex bottle, 7 mg (0.1 equiv.) of Pd(OAc)2, 35 mg (0.4 equiv.) PPh3, 500 mg (1 equiv.) of para-iodo resin in 5 ml DMF were flushed with Argon for 4 minutes. Then, 613 mg (10 equiv.) of tert.-butyl-acrylate and 312 mg (10 equiv.) of NEt3 are added. The mixture is stirred at 80°C for 24 h. The resin is washed 3 times with THF, CH2Cl2, Ether and MeOH and dried in high vacuum.
General Procedure for the Traceless Cleavage:
Due to the effects of different substitution-pattern on the arene-part of the triazene the stability and reactivity of the corresponding diazonium salt can vary to a wide extend. Any acidic reaction condition which favors a radical pathway in THF or Methanol as solvent will lead to a traceless substitution of the diazonium functionality by a hydrogen atom out of the solvent.
The most general method for this transformation is the use of 10% HCl conc. in THF. Per gram resin as obtained above, 10 ml of this solution is added and the resulting slurry is treated with ultrasound in a sonicator bath at 50°C for 5 min. Filtration leads to the compound in high yield. (The resulting solution might contain some polymers resulting from opening of the THF by HCl. These can easily be removed by SPE)
Example 2. Two step Heck type reaction and cleavage Richter reaction.
Heck type alkyne coupling
In a pressure resistant Pyrex bottle, 7 mg (0.1 equiv.) of Pd(OAc)2, 35 mg (0.4 equiv.) PPh3, 500 mg (1 equiv.) of ortho-bromo resin in 5 ml DMF were flushed with Argon for 4 minutes. Then, 613 mg (10 equiv.) of alkyne and 312 mg (10 equiv.) of NEt3 are added. The mixture is stirred at 80°C for 24 h. The resin is washed 3 times with THF, CH2Cl2, Ether and MeOH and dried in high vacuum.
Cleavage Richter reaction
To 100 mg of resin in a Pasteur pipette are added 2 ml 10% HCl/acetone. Then, the resin was rinsed with 6 ml of distilled water into a flask. After stirring for 24 h the mixture is extracted 3 times with 3 ml CH2Cl2. The extract is evaporated in a rotational evaporator and dried in high vacuum.
Example 3. Cleavage Heck reaction
In a 25 ml laboratory bottle, 1 g (0.63 mmol) of o-hydroxymethyl resin is suspended in 5 ml of methanol. Under ice-cooling, 200 µl TFA is carefully added. Then, 0.9 ml (10 mmol) of cyclopentene and 5 mg of palladium acetate is added. This mixture is stirred for 12 h at 50°C. Than the resin was filtrated and the filtrate was evaporated. The purity of the crude product is > 90%.
[1] Part 5 in the series: Nitrogen-based Linker. for part 4, see: S. Bräse, S. Dahmen, J. Heuts, Tetrahedron Lett. 1999, 40, 6201-6203
[2] a) E. M. Gordon, R. W. Barrett, W. J. Dower, S. P. Fodor, M. A. Gallop, J. Med. Chem. 1994, 37, 1385-1401; b) L. A. Thompson, J. A. Ellman, Chem. Rev. 1996, 96, 555-600; c) J. S. Früchtel, G. Jung, Angew. Chem. 1996, 108, 19-46; Angew. Chem. Int. Ed. 1996, 35, 17-42; d) F. Balkenhohl, C. von dem Bussche-Hünnefeld, A. Lansky, C. Zechel, Angew. Chem. 1996, 108, 2437-2487; Angew. Chem. Int. Ed. 1996, 35, 2288-2337; e) P. H. H. Hermkens, H. C. J. Ottenheijm, D. Rees, Tetrahedron 1997, 53, 5643-5678; f) Combinatorial Peptide and Nonpeptide Libraries (Ed. G. Jung), VCH, Weinheim, 1996; g) Combinatorial Chemistry (Eds.: S. R. Wilson, A. W. Czarnik), Wiley-Interscience, New York, 1997, cit. lit.; h) B. A. Bunin, The Combinatorial Index, Academic Press, San Diego 1998, cit. lit..
[3] For reviews about linker strategies: a) B. J. Backes, J. A. Ellmann, Curr. Opin. Chem. Biol. 1997, 1, 86-93; b) I. W. James, Tetrahedron 1999, 55, 4855-4946.
[4] D. Obrecht, J. M. Villalgordo, Solid-Supported Combinatorial and Parallel Synthesis of Small-Molecular-Weight Compound Libraries, Elsevier, Oxford, 1998, p. 98.
[5] a) K. C. Nicolaou, C. N. C. Boddy, S. Natarajan, T.-Y. Yue, H. Li, S. Bräse, J. M. Ramanjulu, J. Am. Chem. Soc. 1997, 119, 3412-3422, cit. lit.; b) K. C. Nicolaou, M. Takayanagi, N. F. Jain, S. Natarajan, A. E. Koumbis, T. Bando, J. M. Ramanjulu, Angew. Chem. 1998, 110, 2881-2883; Angew. Chem. Int. Ed. 1998, 37, 2717, cit. lit..
[6] S. Bräse, D. Enders, J. Köbberling, F. Avemaria, Angew. Chem. 1998, 110, 3614-3616; Angew. Chem. Int. Ed. 1998, 37, 3413-3415.
[7] The T2 linker system 4, 6, 10 and the resin 3 are commercially available (NovaBioChem, Catalogue 1999). http://www.nova.ch. See also innovation sheet 2/99.
[8] S. Bräse, A. de Meijere in Metal-catalyzed Cross-Coupling Reactions, Hrsg. P. J. Stang, F. Diederich. VCH-Wiley. Weinheim. 1998, 99-166.
[9] S. Bräse, M. Schroen, Angew. Chem. 1999, 111, 1139-1142; Angew. Chem. Int. Ed. 1999, 38, 1071-1073.
[10] a) S. Bräse, A. de Meijere, Angew. Chem. 1995, 110, 2881-2883; Angew. Chem. Int. Ed. 1995, 37, 2717, cit. lit..; b) A. de Meijere, H. Nüske, M. Es-Sayed, M. Schroen, S. Bräse, submitted.
[11] H. Zollinger, Diazo Chemistry I. VCH: Weinheim. 1994.
[12] V. von Richter, Ber. Dtsch. Chem. Ges. 1883, 16, 677-683.
[13] For a recent application: S. F. Vasilevsky, E. V. Tretyakov, Liebigs Ann. 1995, 775-779.
[14] a) O. Widman, Ber. Dtsch. Chem. Ges. 1884, 17, 722-727. b) R. Stoermer, H. Fincke, Ber. Dtsch. Chem. Ges. 1909, 42, 3115-3122.
[15] a) W. Borsche, A. Herbert, Liebigs Ann. Chem. 1941, 546, 293-303. b) C. F. Koelsch, J. Org. Chem. 1943, 8, 295-299.
[16] S. Bräse, J. Heuts, S. Dahmen, Tetrahedron Lett. 1999, 40, 6201-6203
[18] For a recent discussion: L. G. Fedenok, I. I. Barabanov, I. D. Ivanchikova, Tetrahedron Lett. 1999, 805-808.
[19] S. Bräse, J. Heuts, unpublished.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0013 as the message subject of your e-mail.