1a-h
[A0002]
Influence of Some Thia or Azasubstituted Butyric Acid Derivatives on Chemical Shift of Carbon Atom of Benzene Ring
Johannes Fröhlich, Sauter Fritz
Institut for Organic Chemistry, Technical
University Wien, Getreidemarkt 9, A-1110 Vienna, Austria
E-mail: [email protected], [email protected]
Department of Organic Chemistry, Faculty of Chemical Technology, Slovak Technical University, Radlinského
9, SK-812 37 Bratislava, Slovak Republic (http://www.chtf.stuba.sk/KATEDRY/koch/milata/Vmhtml.htm)
E-mail: [email protected]
Received: 15 August 1999 / Uploaded: 17 August 1999
Eight thia- or azasubstituted butyric acid derivatives was prepared and influence of
this substituent onto carbon atoms of benzene ring was studied.
KEY WORDS - 4-phenyl-3-thiabutyric acid
derivatives / 4-phenyl-4-thiabutyric acid derivatives / 4-phenyl-3-azabutyric acid
derivatives / substituent chemical shifts
1. INTRODUCTION
2. EXPERIMENTAL SECTION
3. RESULTS AND DISCUSSION
4. REFERENCES
INTRODUCTION
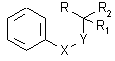
For the synthesis of the derivatives of (iso)thiochromanon and isoquinolone was
necessary to prepare 8 appropriate substituted 4-phenyl-3-(or 4-)thia(or aza)butyric acid
and its derivatives 1:

1a-h
X |
Y |
R1 |
R2 |
R |
X |
Y |
R1 |
R2 |
R |
|||
| 1a | CH2 |
S |
H |
H |
COOH |
1e |
CH2 |
NH |
- ( CH2 )4 - |
CN |
||
| 1b | S |
CH2 |
H |
H |
COOH |
1f |
CH2 |
NH |
- ( CH2 )4 - |
COOH |
||
| 1c | CH2 |
S |
H |
H |
COOMe |
1g |
CH2 |
NMe |
- ( CH2 )4 - |
CN |
||
| 1d | S |
CH2 |
H |
H |
COOMe |
1h |
CH2 |
NMe |
- ( CH2 )4 - |
COOH |
||
EXPERIMENTAL SECTION
The synthesis of thiabutyric acid derivatives was long ago described [1-3] but their 13C
NMR spectra were till now not published. In the case of 3-azabutyronitrile derivatives was
applied the modified Strecker's method [4] for the synthesis the corresponding
2,2-tetramethylene analogs according lit. [5]. NMR spectra were determined with a Bruker
AC 200 FT-NMR spectrometer and are expressed in ppm downfield to tetramethylsilane
(internal standard).
4-Phenyl-3-aza-2,2-tetramethylene acid derivatives 1
To a mixture of 20 mmol N-(un)substituted benzylamine, 20 mmol hydrochloric acid, 40 ml of
25 % ethanol, 20 mmol cyclopentanone and 20 ml of ethanol was dropped a solution of 30
mmol kalium cyanide in 10 ml of water under magnetic stirring. After 4 days was the
precipitate (1e) filtered off or was the reaction mixture (1g) extracted
with ether , dried with sodium sulfate and evaporated. Hydrolysis of the nitriles to
corresponding acids was occured with concentrated sulfuric acid by 20-100°C during 12
hours and than worked-up after neutralisation and filtration or extraction, drying and
evaparation. 1e: 62 %, 42-3 °C; 1g: 76 %, 130-140 °C / 0.45 Torr.
RESULTS AND DISCUSSION
Chemical shifts of all of the studied compounds were established on the basis of comparison of the experimental values of the chemical shifts acquired from recorded spectra and calculated ones using the following programs: ACD / CNMR ver. 1.1 [6], CS Chem Draw Pro, ver. 4.5 [7] and ChemWindow3, ver. 3.0.0 with C13-module [8], respectively. We obtained a good accordance of the measured and calculated values of the chemical shifts obtained using all three programs.
Chemical shifts (CS) and a substituent chemical shift (SCS) of all of the compounds with heteroatom in b-position are in the same range for all compounds. The compounds with heteroatom in a-position (1b,d) have the ipso-carbon not so much shifted like the analogues having heteroatom in b-position (1a,1c,1e-1h). Substuituent chemical shifts of meta-carbon atoms of the benzene ring of compounds 1a, 1b and 1d have positive value unlike the all others compounds 1c, 1e-1h.
Influence of the studied substituents on chemical shifts of carbon atoms of the benzene ring is possible to compare for example with CH2SMe (1a, 1c), SMe (1b, 1d) or CH2NHCHMe2, CH2NMeCH2Ph substituents [9] with good agreement with model compounds. Perhaps SCS for Cipso are slight reduced for all compounds for about 2 ppm and 1b, 1d have positive values for Cortho in comparison with SMe substituent, but this is also depending from solvents used [9]..
Chemical shifts (CS, in d ppm) and substituent chemical shifts (SCS) of carbon atom of benzene ring of 4-phenyl-3-(or 4-)thia(or aza)butyric acid derivatives
- X - Y - CR1R2R* |
Cipso |
Cortho |
Cmeta |
Cpara |
Other signals | |
| - CH2-S-CH2-COOH, 1a | CS |
136.8 |
129.2 |
128.6 |
127.4 |
176.8, 36.3, 31.9 |
SCS |
+8.3 |
+0.7 |
+0.1 |
-1.1 |
||
| -S-CH2CH2-COOH, 1b | CS |
135.0 |
130.1 |
129.0 |
126.6 |
177.1, 34.4, 30.9 |
SCS |
+6.5 |
+1.6 |
+0.5 |
-1.9 |
||
| -CH2-S-CH2-COOMe, 1c | CS |
137.0 |
128.9 |
128.3 |
127.0 |
170.5, 52.0, 36.1, 31.8 |
SCS |
+8.5 |
+0.4 |
-0.2 |
-1.5 |
||
| -S-CH2CH2-COOMe, 1d | CS |
135.0 |
129.8 |
128.8 |
126.3 |
171.8, 51.5, 33.9, 28.7 |
SCS |
+6.5 |
+1.3 |
+0.3 |
-2.2 |
||
| -CH2-NH-C(CH2)4CN, 1e | CS |
139.2 |
128.5 |
128.3 |
127.3 |
122.9, 61.2, 50.2, 39.0, 23.5 |
SCS |
+10.7 |
0 |
-0.2 |
-1.2 |
||
| -CH2-NH-C(CH2)4COOH, 1f | CS |
140.0 |
128.4 |
127.7 |
127.0 |
179.5, 70.0, 48.4, 35.8, 24.2 |
SCS |
+11.5 |
-0.1 |
-0.8 |
-1.5 |
||
| -CH2-NMe-C(CH2)4CN, 1g | CS |
138.3 |
128.5 |
128.2 |
126.8 |
120.2, 68.8, 58.4, 38.5, 37.9, 23.2 |
SCS |
+9.8 |
0 |
-0.3 |
-1.7 |
||
| -CH2-NMe-C(CH2)4COOH, 1h a | CS |
140.0 |
128.3 |
128.2 |
126.7 |
176.6, 74.7, 56.7, 36.0, 32.1, 24.8 |
SCS |
+11.5 |
-0.2 |
-0.3 |
-1.8 |
*d (Benzene) = 128.5 ppm, d (Chloroform) = 77.0 ppm, ain DMSO-d6, d (DMSO) = 39.0 ppm
REFERENCES
1. J.C. Petropoulos, M.A. McCall,D.S. Tarbel, J.Amer.Chem.Soc. 75, 1133 (1953).
2. A.K. Kiang, F.G.. Mann, J.Chem.Soc. 1951, 1911.
3. H.O. Fong, W.R. Hardstaff, D.G. Kay, R.F. Langler, R.H. Morse, D.-N. Sandoval, Can.J.Chem. 57(10), 1206 (1979).
4. A. Strecker, Justus Liebigs Ann.Chem. 75, 27 (1850).
5. M.R. Euerby, R.D. Waigh, J.Chem.Res.(M) 1982, 2417.
6. ACD / CNMR: Advanced Chemistry Development Inc., 141 Adelaide Street West Suite 1501, Toronto, Ontario, Canada M5H 3L5
7. CS Chem Draw Pro: CambridgeSoft Corporation, 875 Massachusetts Avenue, Cambridge, MA 021 39 USA
8. ChemWindow3: SoftShell Internationall, 715 Horicon Dr Ste 390, Grand Junction, CO 815 06 USA
9. D.F. Ewing, Org.Magn.Reson. 12(9), 499 (1979).
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0002 as the message subject of your e-mail.