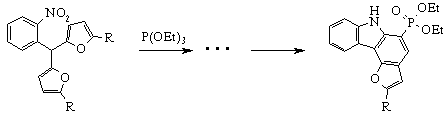
[A0004]
INDOLE, CINNOLINE, BENZOTHIAZINE AND
DIHYDROFUROQUINOLINE DERIVATIVES FROM ARYLDIFURYLMETHANES
Alexander V. Butina, Vladimir T. Abaevb, Tat'yana A. Stroganovaa, Andrey V. Gutnovb, Irina V. Lodinaa and Olga V. Shurakova.
a Research Laboratory of Furan Chemistry, Kuban State Technological
University,
Moskovskaya 2, Krasnodar, 350072 Russia
E-mail: [email protected]; [email protected]
b
Department of Organic Chemistry, North Ossetian University, Vatutina st., 46, Vladikavkaz, 362025, RussiaE-mail: [email protected], [email protected]
Received: 30 July 1997 / Uploaded: 6 August 1997
INTRODUCTION
Until recently the most publications on furylarylmethanes were devoted to their application as promising source for polymer producing [1-3] or as intermediates in the synthesis of calixarenes and crown ethers [4, 5].
At the same time, due to the availability of furan ring in the molecules of these compounds they can serve as convenient and useful precursors for syntheses of various heterocyclic compounds condensed with benzene cycle.
But looking through the literature we could find only one example of similar use of furylarylmethanes: Prof. Gurnos Jones and co-workers obtained carbazole derivative from 2-nitrophenyldifurylmethane under treatment of latter with triethylphosphite [6].
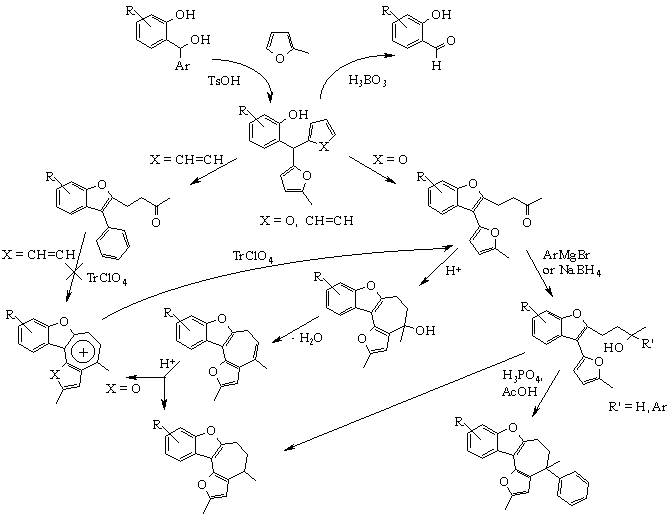
Over several years we have developed new approaches to synthesis of novel heterocyclic system based on compounds of furylarylmethane series. We carefully investigated condensation of different salicylaldehydes with sylvan caused benzofuran and oxazulene derivatives formation [7]. This reaction is given on Scheme 1:
Continuing the investigations on use of aryldifurylalkanes as synthons, in this report we would like to present our new results on syntheses of various nitrogen-containing heterocycles from furylarylmethanes.
INDOLES
To synthesize novel nitrogen-containing heterocyclic compounds we decided to get 2-aminoaryldifurylmethanes by the reduction of corresponding 2-nitroaryldifurylmethanes.
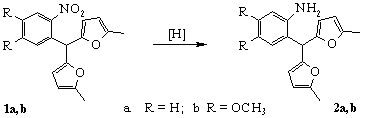
1) NaBH4, Pd/C;
2) NH2NH2 . H2O, Pd/C, FeCl3, MeOH;
3) Zn, Me3SiCl, MeOH
At the reduction of compounds 1a,b in methanol by zinc dust in the presence of hydrochloric acid as well as with hydrazine hydrate - Pd/C system, corresponding anilines 2a,b were obtained in good yields.
But we observed different results in the SnCl2 promoted reduction of the compounds 1a,b in acidic media. o-Nitrophenyldifurylmethane 1a reduction led to the formation of indole derivative 3 instead of expected aniline 2a (Scheme 2) [8].
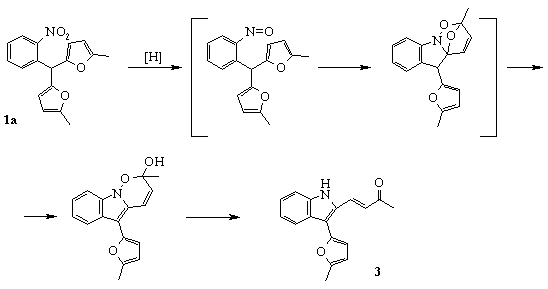 Scheme 2
Scheme 2
We suggested that the key intermediate of this transformation was o-nitrozophenyldifurylmethane, and the main stage was cycloaddition between nitrozo-group and one of furan rings.
Compound 1b was reduced into corresponding aniline 2b under this condition. It could be explained with deactivated influence of electron-donating substituents on nitrozo-group.
The described approach has essential disadvantage: electron-donating substituents in benzene ring hinder the heterocycloaddition and indole formation.
Taking into account the great practical importance of indole derivatives we have tried to develop the universal approach to the synthesis of these compounds. Considering the similar chemical behaviour of phenolic hydroxy- and acylaminogroup in aromatic compounds we suggested to synthesise indole derivatives using the mechanism proposed earlier for benzofuran derivatives [9]. For this purpose 2-acylaminoaryldifurylmethanes 4a,b were obtained. Under treatment with trityl perchlorate they were transformed into tetracyclic salts 5a,b:
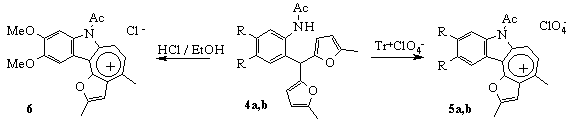
It should be noted that expected 2-(3-oxobutyl)-3-furylindoles were not detected under treatment of compounds 4a,b with ethanolic solution of hydrogen chloride. In case R = OCH3 (4a) we isolated salt 6. Its formation can be explained with strong electron-donating effect of indole fragment on furan ring, what, in its turn, facilitates the secondary cyclization.
In our recent study we showed that replacing of one of furan cycles in 2-hydroxyaryldifurylmethanes with aromatic ring retarded the tetracyclic salt formation because of the sterical strain [10].
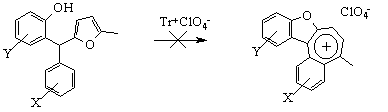
On this basis, we have synthesised the corresponding 2-acylaminoaryl(aryl)furyl-methanes 7 and rearranged them into indole derivatives 8 under acidic conditions.

At present this study is in progress.
CYNNOLINES
The other example showing the application of aylfurylmethanes as synthones for the preparation of novel heterocyclic compounds is the synthesis of cynnoline derivatives as a result of the intramolecular electrophylic attack of diazonium group to furan ring. Earlier attempts to obtain cynnolines were failed due to strong resinification of a reaction mixture under the action of aqueous solutions of NaNO2 and HCl [6]. Carrying out the diasotation reaction in the mild conditions (isoamylnitrite, Me3SiCl, acetonitrile), we succeded to obtain cynnoline derivatives 9 in high yield [8].
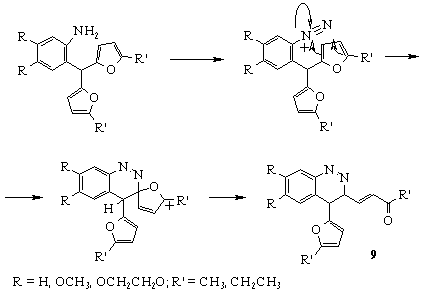
BENZOTHIAZINES
Using a standard procedure, we have obtained isothiocyanates 10 which under the action of perchloric acid were transformed into 4H-bensothiazines-3,1 11. The possible mechanism of this conversion is given on Scheme 3 and, in our opinion, it includes intramolecular ipso-substitution of one of furan cycles by protonated isothiocyano-group with subsequent alkylation of sulfur-atom by a carbenium cation, arising from C-C bond fission [8].
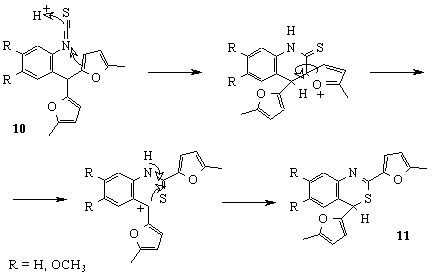
Scheme 3
This study is being continued.
DIHYDROFURO[3,2-b]-QUINOLINES
Carrying on our investigations of 2-nitroaryldifurylmethanes we have established that under irradiation with ultraviolet light they are converted into 4,9-dihydrofuro[3,2-b]-quinoline derivatives 12 [11] (Scheme 4).
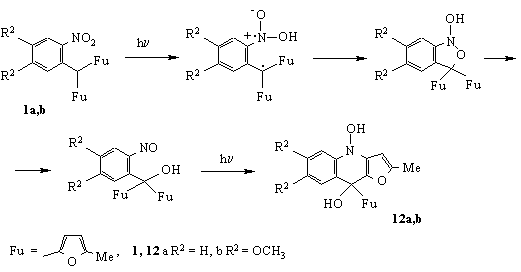
CONCLUSION
So, in our opinion, the indicated results open new possibilities in the difurylarylmethanes application as synthons for new condensed polycyclic heterocyclic compounds synthesis.
REFERENCES
[1] Boufi, S.; Gandini, A.; Belgacem, M. Polymer 1995, 36, 1689.
[2] He, X.; Conner, A. H.; Koutsky, J. A. J. Polym. Sci., Part A: Polym. Chem. 1992, 30, 533.
[3] Cawse, J. L.; Stanford, J. L.; Still, R. H. Makromol. Chem. 1984, 185, 709.
[4] Brown W.H., Hutchinson B.J. Can. J. Chem., 1978, 56, 617.
[5] Musau R.M., Whiting A. J. Chem. Soc., Chem. Commun. 1993, 12, 1029.
[6] Jones, G.; McKinley, W. H. J. Chem. Soc., Perkin Trans. 1. 1979, 599.
[7] Butin A.V., Gutnov A.V., Abaev V.T., Krapivin G.D. Molecules 1999, 4,
[8] Butin, A. V.; Abaev, V. T.; Stroganova, T. A.; Gutnov, A. V. Molecules 1997, 2, 62.
[9] Butin, A. V.; Abaev, V. T.; Zavodnik, V. E.; Kul’nevich, V. G. Khim. Geterotsikl. Soedin. 1993, 627.
[10] Gutnov A.V., Butin A.V., Abaev V.T., Krapivin G.D. Molecules, in press.
[11] Stroganova T.A., Butin A.V., Abaev V.T., Kul'nevich V.G. Khim. Geterotsikl. Soedin. 1998, 9, 1250.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0004 as the message subject of your e-mail.