

[A0007]


1,3-Dipolar Cycloadditions of N-Benzyl Furfuryl Nitrones to Vinyl Ethers and a,b-Unsatureted Esters.
T. Tejero, S. Anoro, F.L. Merchan and P. Merino
Laboratorio
de Sintesis Asimetrica, Departamento de Quimica
Organica, Facultad de Ciencias-ICMA, Universidad
de Zaragoza-CSIC, E-50009 Zaragoza, Aragon,
Spain.
E-mail: [email protected]
Received: 21 July 1997 / Uploaded: 5 August 1997
Introduction
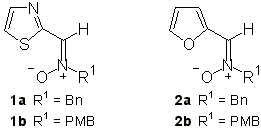
While abundant information on the reactivity of several classes of nitrones exists [1], hetaryl nitrones, i.e. nitrones bearing an heterocyclic ring at the carbon atom, have been the subject of very few investigations [2]. Recent publications from our laboratory have been concerned with the use of thiazolyl nitrones 1 as a suitable substrate for both nucleophilic additions [3] and 1,3-dipolar cycloadditions [4]. Our continued interest in the reactivity of hetaryl nitrones has led to investigate the reaction of the furfuryl nitrones 2 with several alkenes. In this communication we report our preliminary results on this topic.
Scheme 1
Results
Furan-derived nitrones were prepared according to our method [5] from furfural and the corresponding benzyl hydroxylamine. In both cases only the Z-isomer was detected, the configuration being assigned on the basis of NOE experiments (a 10-12% enhancement was observed in the difference spectra upon irradiation of the azomethine proton of the nitrone and the benzylic protons. Further confirmation arose from the registration of the corresponding 1-H NMR spectra in deuterochloroform and hexadeuterobenzene. The observed ASIS effect [6] confirmed the Z-configuration of nitrone 2. The configurational stability of nitrones 2 was also checked. After refluxing in toluene for a week no changes in their structure were observed and the E-isomer could not be detected in any instance.
Refluxing nitrones 2 either in a solvent or neat with an excess of alkene 3 until TLC indicated disappearence of the nitrone afforded a mixture of isoxazolidines (Scheme 2). The obtained crude mixture was analyzed by NMR to determine the isomers ratio, and the corresponding adducts were separated by flash chromatography. The isoxazolidines obtained are indicated in Scheme 2 and the corresponding reaction conditions, selectivities and yields in Table 1.
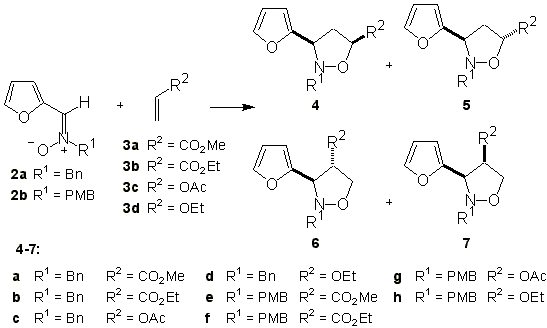
Scheme 2
Table 1. Cycloadditon of nitrones 2 with alkenes 3
entry |
R1 |
R2 |
reaction conditions |
4 |
5 |
6 |
7 |
yield(%) |
1 |
Bn |
CO2Me |
neat / reflux / 5h |
17 |
70 |
13 |
0 |
80 |
2 |
Bn |
CO2Me |
DCM / reflux / 48 h |
40 |
54 |
6 |
0 |
60 |
3 |
Bn |
CO2Me |
DCE / reflux / 16 h |
28 |
58 |
14 |
0 |
96 |
4 |
Bn |
CO2Me |
Toluene / reflux / 12 h |
22 |
58 |
20 |
0 |
68 |
5 |
Bn |
CO2Me |
CHCl3 / reflux / 24 h |
36 |
50 |
14 |
0 |
93 |
6 |
Bn |
CO2Et |
DCE / reflux / 16 h |
16 |
67 |
13 |
0 |
75 |
7 |
Bn |
CO2Et |
Toluene / reflux / 12 h |
27 |
73 |
0 |
0 |
68 |
8 |
Bn |
OEt |
Toluene / reflux / 10 d |
40 |
60 |
0 |
0 |
25 |
9 |
Bn |
OAc |
CHCl3 / reflux / 10 d |
12 |
88 |
0 |
0 |
36 |
10 |
PMB |
CO2Me |
Toluene / reflux / 12 h |
22 |
61 |
17 |
0 |
96 |
11 |
PMB |
CO2Me |
DCE / reflux / 16 h |
19 |
64 |
17 |
0 |
91 |
12 |
PMB |
CO2Me |
CH2Cl2 / reflux / 9 d |
20 |
60 |
20 |
0 |
85 |
13 |
PMB |
CO2Me |
neat / reflux / 5h |
10 |
82 |
8 |
0 |
91 |
14 |
PMB |
CO2Et |
Toluene / reflux / 16 h |
27 |
63 |
10 |
0 |
56 |
15 |
PMB |
CO2Et |
DCM / reflux / 9 d |
20 |
69 |
11 |
0 |
85 |
16 |
PMB |
OEt |
Toluene / reflux / 13 d |
38 |
62 |
0 |
0 |
53 |
17 |
PMB |
OAc |
CHCl3 / reflux / 17 d |
40 |
60 |
0 |
0 |
16 |
18 |
PMB |
OAc |
neat / reflux / 10 d |
35 |
65 |
0 |
0 |
43 |
Bn: benzyl; PMB: p-methoxybenzyl; DCM: dichloromethane; DCE: 1,2-dichloroethane
The cycloaddition of nitrones 2 with disubstituted alkenes, e.g. dimethyl fumarate and dimethyl maleate was also studied. The results of this study are illustrated in Scheme 3 and summarized in Table 2.
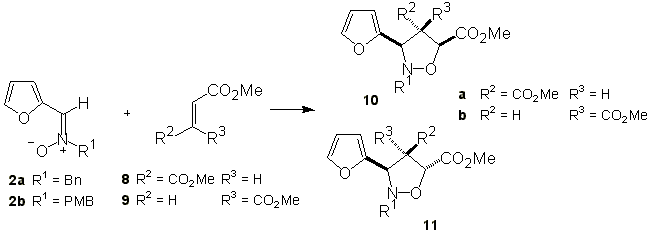
Scheme 3
Table 2. Cycloaddition of nitrones 2 with alkenes 8-9.
entry |
R1 |
R2 |
R3 |
reaction conditions |
10 |
11 |
yield(%) |
1 |
Bn |
CO2Me |
H |
DCM/ reflux / 48 h |
47 |
53 |
91 |
2 |
Bn |
CO2Me |
H |
Toluene / reflux / 12 h |
61 |
39 |
54 |
3 |
Bn |
H |
CO2Me |
1,2-DCE / reflux / 16 h |
81 |
19 |
79 |
4 |
Bn |
H |
CO2Me |
Toluene / reflux / 12 h |
82 |
18 |
89 |
5 |
PMB |
CO2Me |
H |
Toluene / reflux / 12 h |
93 |
7 |
86 |
6 |
PMB |
H |
CO2Me |
Toluene / reflux / 12 h |
85 |
15 |
83 |
Bn: benzyl; PMB: p-methoxybenzyl; DCM: dichloromethane; DCE: 1,2-dichloroethane
The structure and stereochemistry of isoxazolidines 4-7 were ascertained by careful examination of the 1-H NMR spectra (using, when necessary, homonuclear proton NMR decoupling) and nuclear Overhausser effect (NOE) experiments. Differentiation of 3,5- and 3,4- regioisomers was made on the basis of multiplicity for H-3 (isoxazolidine numbering). Compounds 4 and 5 showed the same 1-H NMR trend for H-3 and H-5 protons that allowed the assignment of the regiochemistry. Both compounds showed a doublet-of-doublets for H-3 whereas compound 6 (the only 3,4-regioisomer obtained) showed a doublet for the same proton (Figure 1). In addition compounds 6 displayed two vicinal hydrogen atoms (coupling constants J>12 Hz) which were assigned to the H-5a and H-5b protons.The alternative 3,5-regioisomers 4-5 bearing the R2 substituent at carbon C-5 showed two vicinal hydrogen atoms at higher fields than those corresponding to H-5a and H-5b in 6, thus being assigned to H-4a and H-4b.
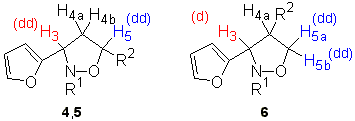
Figure 1. Determination of the regioselectivity
The analysis of vicinal coupling constants J(3,4) and J(4,5)did not result in an unambiguous configurational assignment of compounds 4-6. We have used nuclear Overhausser effects obtained by difference spectroscopy experiments for establishing the relative stereochemistry of the ring substituents for isoxazolidines 4-6 (Figure 2). The irradiation of H-4b in 4 resulted in enhancement of the signals for H-3 and H-5. On the other hand, no effect was detected upon irradiation of H-4b. The same experiment performed on products 5 produced signal enhancements for H-3 when H-4b was irradiated and for H-5 upon irradiations of H-4a. The irradiation of H-3 of compound 6 showed an enhancement of the signals for H-4 thus suggesting that the two protons are spatially close. Similarly, H-4 was also affected upon irradiation of H-5b and viceversa.
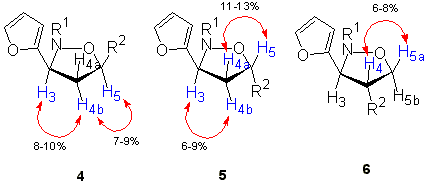
Figure 2. Determination of the stereoselectivity
All these effects are in agreement with a cis configuration for compounds 4 and
a trans configuration for both 5 and 6. The configuration of compounds 10
and 11 was also established by NOE experiments.
Discussion
The stereochemical outcome of the cycloadditions did not appear to be affected by the electron density of the dipolarophile. Both with electron-rich (Table 1, entries 1-7 and 10-15) and electron-deficient (Table 1, entries 8-9 and 16-18) alkenes similar results were obtained. In all cases the 3,5-regioisomers were obtained as major adducts. This observed regioselectivity is in agreement with previously reported data for other nitrones [7] and it can be deduced from a frontier molecular orbitals treatment in which the HOMO-nitrone LUMO-alkene interactions dominate. Only in the case of cycloaddition with ethyl vinyl ether the interactions LUMO-nitrone HOMO-alkene are more favourable [8]. In this case the smallest HOMO-LUMO gap exists for the LUMO of the nitrone and the HOMO of the alkene. The energies and the coefficients of the HOMO and LUMO of nitrones 2 and alkenes 3 calculated at a semiempirical level (MOPAC97, PM3) [9] were shown in Figures 3 and 4, respectively. The observed regioselectivity can be explained by the magnitude of the atomic components in the FMO of interest. Thus, for cycloadditions with methyl acrylate 3a and vinyl acetate 3b the atom with the calculated larger HOMO coefficient on the nitrone reacts with the atom with the larger LUMO coefficient of the alkene. On the other hand, in the cycloaddition of nitrones 2 with ethyl vinyl ether 3c the atom with the calculated larger LUMO coefficient on the nitrone reacts with the atom with the larger HOMO coefficient of the alkene. The PM3-calculated FMO energies and atomic contributions are also given in Table 3.

Figure 3. PM3-calculated FMO energies and atomic contributions for nitrones 2
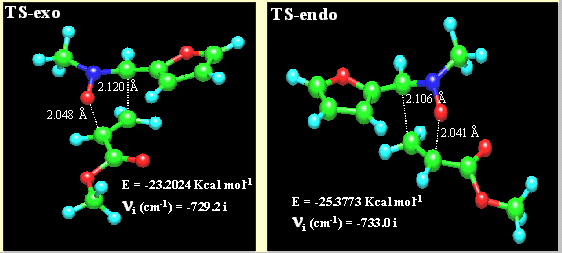
Figure 4. PM3-calculated FMO energies and atomic contributions for alkenes 3
Table 3. PM3-calculated FMO energies and atomic contributions (favourable in blue)
HOMO-nitrone |
LUMO-nitrone |
||||||||||
LUMO-alkene |
2a |
-8.3984 |
2b |
-8.3707 |
HOMO-alkene |
2a |
-0.3373 |
2b |
-0.2869 |
||
3a |
-0.0832 |
8.3152 |
8.2875 |
3a |
-11.0669 |
10.7296 |
10.7800 |
||||
3b |
0.5804 |
8.9788 |
8.9511 |
3b |
-9.9511 |
9.6138 |
9.6642 |
||||
3c |
1.3292 |
9.7276 |
9.7000 |
3c |
-9.4589 |
9.1216 |
9.1720 |
||||
To discuss the stereoselectivities observed in the 1,3-dipolar cycloadditions studies it is required a careful evaluation of the different effects which can operate in the transition states leading to two diastereomeric cis and trans cycloadducts. The possibility of interconversion of the E and Z forms of the nitrones, although suggested for several authors [10], can be discarded on the basis of the proved stability of compounds 2 (vide supra). The preferential formation of trans isoxazolidines 5 can be explained by assuming an endo-approach of the dipolarophile to the nitrone. In order to corroborate this hypothesis and once assumed the preferred formation of 3,5-regioisomers, we carried out a PM3 analysis of the cycloaddition reaction between nitrone 2a (in which the benzyl group was replaced by a methyl group) and methyl acrylate. PM3 methods have been successfully used by other authors to explain the stereochemical results of 1,3-dipolar cycloaddtions[11].
The theoretical study included the starting system, the transition state and the primary cycloadduct for both endo and exo approaches. The more stable conformation was chosen for nitrone and alkene. The PM3 calculated heats of formation of nitrone and alkene were 9.049 Kcal/mol and –67.4557 Kcal/mol, respectively. Sum of these values (-58.407 Kcal/mol) was considered to be the heat of formation of the reactants. The heat of formation of cis-isoxazolidine (coming from an exo approach) was –87.6031 Kcal/mol and that of the trans cycloadduct (coming from an endo approach) was –87.9558 Kcal/mol. The optimized transition structures are illustrated in Figure 5 and the PM3-calculated parameters are given in Table 3. By intrinsic reaction coordinate calculation the transition states, the reagents and the corresponding cycloadducts were confirmed to be in the same reaction coordinate. According to the data collected in Table 4 the TS-exo involves 35.20 Kcal/mol of activation enthalpy, significantly greater than that of TS-endo (33.03 Kcal/mol). These results are in good agreement with the experimental data.
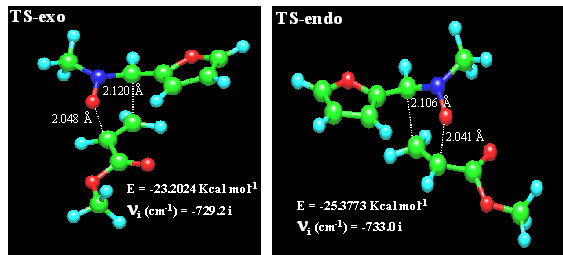
Figure 5. PM3-calculated transition states
Table 4. PM3-calculated selected data
compound |
Heat of formation |
nitrone |
9.0490 |
methyl acrylate |
-67.4557 |
TS-exo |
-23-2024 |
TS-endo |
-25.3773 |
cis-isoxazolidine |
-87.6031 |
trans-isoxazolidine |
-87.9558 |
In conclusion, our studies have revealed that the 1,3-dipolar cycloaddition of
C-furfuryl-N-benzyl nitrones with alkenes gives predominantly trans-3,5-disubstituted
isoxazolidines. The regiochemistry of the cycloaddition seems to be controlled by FMO
interactions, whereas the stereochemistry of the cycloaddition is mainly domionated by the
preference for an endo approach. With these results in hand, we are now expanding
this reaction to other hetaryl nitrones and applying it to the synthesis of several
nitrogenated compounds.
References and Notes
[1] (a) Torssell, K.G.B. Nitrile oxides, nitrones and nitronates in Organic Synthesis; VCH: New York, 1988. (b) Tuffariello, J.J. 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley: New York, 1984; Vol. 12, p. 83. (c) Gothelf, K.V.; Jorgensen, K.A. Chem. Rev. 1999, 99, 863. (d) Frederickson, M. Tetrahedron 1997, 53, 403. (e) Confalone, P.N.; Huie, E.M. Org. React. 1988, 36, 1. (f) Padwa, A. In Comprehemsive Organic Synthesis, Trost, B.M.; Fleming, I., Eds.; Pergamon: Oxford, 1991, Vol. 4, p. 1069. (g) Wade, P.A. In Comprehemsive Organic Synthesis, Trost, B.M.; Fleming, I., Eds.; Pergamon: Oxford, 1991, Vol. 4, p. 1111. (h) Chiacchio, U.; Resciffina, A.; Romeo, G. In Targets in Heterocyclic Systems, Attanasi, O.; Spinelli, D., Eds.; Italian Chemical Society: Roma, 1997, Vol.1, p. 225. (i) Merino, P.; Tejero, T. Molecules 1999, 4, 165 and references cited therein.
[2] For some particular example see: (a) Camiletti, C.; Poletti, L.; Trombini, C. J. Org. Chem. 1994, 59, 6843. (b) Basha, A.; Henry, R.; McLaughlin, M.A.; Ratajczyk, J.D.; Wittenberger, S.J. J. Org. Chem. 1994, 59, 6103. (c) Moriyama, S.; Vallee, Y. Synthesis 1998, 393.
[3] Merchan, F.L.; Merino, P.; Rojo, I.; Tejero, T.; Dondoni, A. Tetrahedron: Asymmetry 1996, 7, 667.
[4] Tejero, T.; Dondoni, A.; Rojo, I.; Merchan, F.L.; Merino, P. Tetrahedron 1997, 53, 3301.
[5] Dondoni, A.; Franco, S.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Synth. Commun. 1994, 24, 2537
[6] Aurich, H.-G.; Franzke, M.; Keiselheim, H.P. Tetrahedron 1992, 48, 663.
[7] (a) Jorgensen, K.A.; Gothelf, K.V. Acta Chem. Scand. 1996, 50, 652. (b) Cordero, F.M.; Aniquini, B.; Goti, A.; Brandi, A. Tetrahedron 1993, 49, 9867. (c) Bimanand, A.Z.; Houk, K.N. Tetrahedron Lett. 1983, 24, 435. (d) Dicken, C.M.; DeShong, P. J. Org. Chem. 1982, 47, 2047. (e) DeShong, P.; Dicken, C.M.; Staib, R.R.; Freyer, A.J.; Weinreb, S.M. J. Org. Chem. 1982, 47, 4397. (f) Cristina, D.; DeMicheli, C.; Gandolfi, R. J. Chem. Soc. Perkin Trans 1 1979, 2891.
[8] Collins, I.; Nadin, A.; Holmes, A.B.; Long, M.A.; Man, J.; Baker, R. J. Chem. Soc. Perkin Trans 1 1994, 2205.
[9] PM3 calculations were carried out using MOPAC 97 as implemented in the WINMOPAC 2.0 package (Fujitsu, 1998).
[10] Inter alia: (a) Rispens, M.T.; Keller, E.; Lange, B.; Zijlstra, R.W.J.; Feringa, B.L. Tetrahedron: Asymmetry 1994, 5, 607. (b) Chiacchio, U.; Casuscelli, F.; Corsaro, A.; Rescifina, A.; Romeo, G.; Uccella, N. Tetrahedron 1994, 50, 6671. (c) Chiacchio, U.; Buemi, G.; Casuscelli, F.; Procopio, A.; Rescifina, A.; Romeo, G. Tetrahedron 1994, 50, 5503.
[11] (a) Raimondi, L. Gazz. Chim. Ital. 1997, 127, 167. (b) Tanaka, K.; Imase, T.; Iwata, S. Bull. Chem. Soc. Jpn. 1996, 69, 2243. (c) Annunziata, R.; Benaglia, M.; Cinquini, M.; Cozzi, F.; Raimondi, L. J. Org. Chem. 1995, 60, 4697.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0007 as the message subject of your e-mail.