[A0009]
Sulfidation of Heterocyclic 1,3-Dicarbonyl Systems
Barbara Schnell and Thomas Kappe
Institute of Organic Chemistry, Karl-Franzens-University Graz, A-8010 Graz, Austria.
Fax: ++43-316-3809840 E-mail: [email protected]
and [email protected]
Received: 27 July 1999 / Uploaded: 6 August 1999
Abstract
Anions of heterocyclic five-or six-membered 1,3-dicarbonyl systems react
with aromatic disulfides and other -S-S- systems, such as dithiuramdisulfides in DMF in
the presence of potsssium carbonate to yield sulfides. The heterocyclic systems studied
include pyrazol-1,3-diones, barbituric acid [1], 6-hydroxy-3(2H)-pyridazinones [2],
4-hydroxy-2-pyrones, 4-hydroxy-2-pyridones, and their benzo derivates (e.g. coumarins and
2-quinolones [3]).
Oxidation of the aromatic sulfides with peracids yields sulfones, while careful oxidation
with hydrogenperoxide in alkaline medium affords the sulfoxides. The latter class of
compounds are heteroanalogs of the well known cyclic tricarbonyl methane derivatives (SO
instead of CO) which show a broad range of biological activity, especially in agricultural
chemistry.
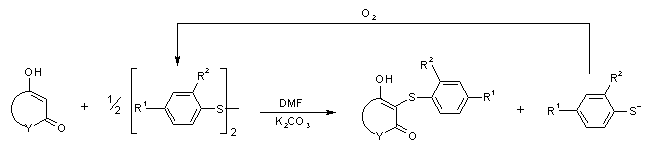
General Scheme for the Preparation of Aromatic
Sulfides from Heterocyclic 1,3-Dicarbonyl Systems (in DMF/ potassium carbonate) 
Note that the thiolate anion is easily oxidized by oxygen (air) to yield the
aromatic disulfide back. Thus only 0.5 equivalents of the aromatic disulfide is needed.
General Scheme for the Oxidation of Aromatic Sulfides
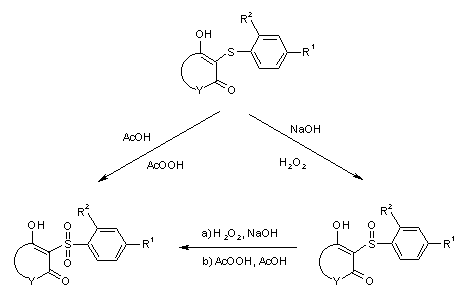
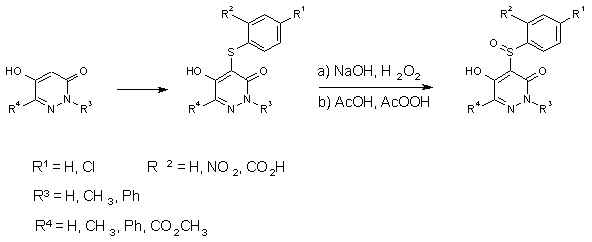
The oxidation with hydrogenperoxide in dilute sodium hydroxide solution yields usually the
sulfoxides while oxidation under more drastic conditions, e.g. with peracids in acetic
acid leads to the sulfones.
Synthesis of the Starting Disulfides
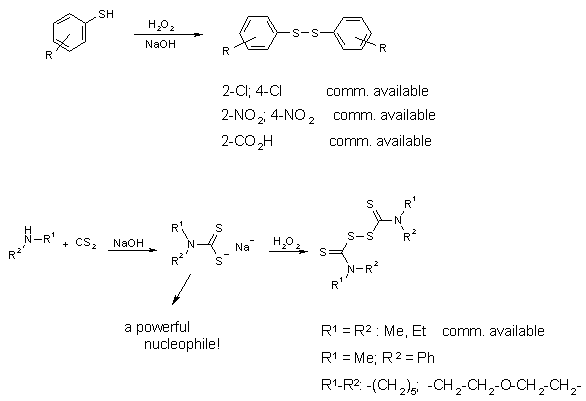
Most of the aromatic disulfides used in our study are commercially available. If not,
they can quantitatively be obtained by oxidizing the alkaline solution of the
thiophenolates with hydrogenperoxide. The thiuram disulfides can be obtained by oxidizing
the solution prepared from secondary amine, sodium hydroxide and carbondisulfide with
hydrogenperoxide.
Sulfidation of 4-Hydroxy-2-pyrones and
4-Hydroxy-2-pyridones and Oxidation of the Thioethers obtained 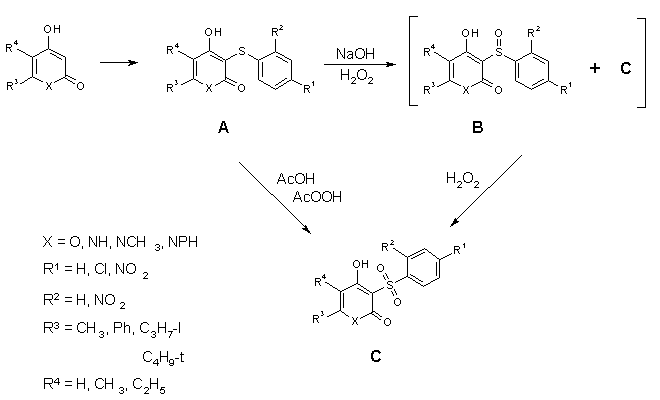
Sulfidation and Subsequent Oxidation of
4-Hydroxy-coumarins and 4-Hydroxy-2-quinolones [3] 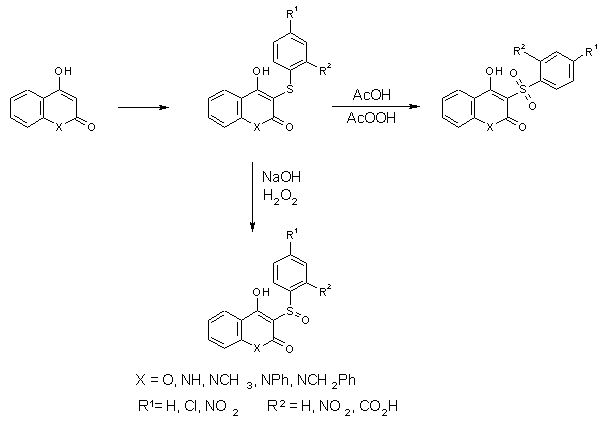
Sulfidation and Oxidation of
5-Hydroxy-3(2H)-pyridazinones [2]
Reaction Products obtained from 6-Hydroxy-4-pyrimidones,
4-Hydroxy-5,6,7,8-tetrahydro-2-quinolones,
2-Hydroxy-pyridopyrimidin-4-ones and 5-Hydroxy-3-pyrazolones
(„Pyrazoldiones“, as Examples of 5-Membered Ring Systems) 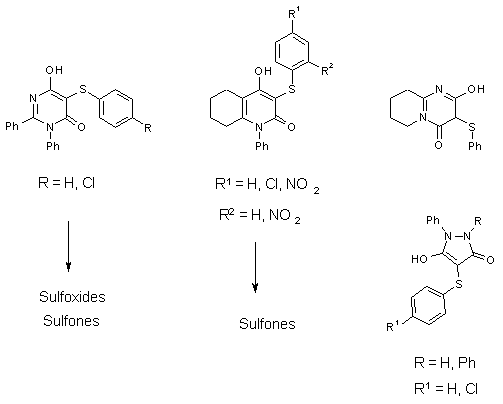
Synthesis of Aliphatic Sulfides and Their Oxidation
Products
Aliphatic thioesters cannot be obtained by this method. The aliphatic disulfides are not
electrophilic enough. They are available by the reaction of
3-chloro-4-hydroxy-2-quinolones with the corresponding alkylthiol. 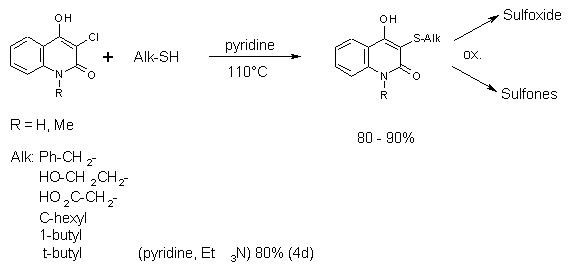
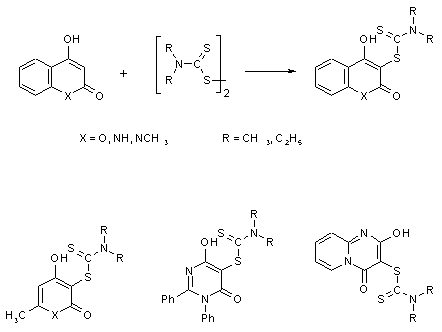
The Reaction of Heterocyclic 1,3-Dicarbonyl Systems with Dithiuram-disulfides
In these reaction one equivalent of the disulfide is needed. No
reoxidation of the produced anion with air is possible (hydrogenperoxide would be
necessary, see synthesis of disulfides)
[1] O.Y. Neilands, I. Sudmale, B. Schnell, K. Georgieva and Th. Kappe, J.
Heterocyclic. Chem., 35, 157 (1998).
[2] Th. Kappe:: "Pyridazines Functionalized in Position 3 and 5 with
Heteroatoms" (Review). J. Heterocyclic. Chem. 35, 1111-1122 (1998).
[3] B. Schnell and Th. Kappe, Monath. 1999 in press.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0009 as the message subject of your e-mail.