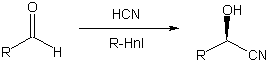The cyanohydrins obtained are known to possess the R-configuration,
provided that the hydroxyl substituent has priority over substituent R. A wide variety of
substrates can be employed in the R-Hnl catalyzed reaction. These include aromatic,
heterocyclic, and saturated as well as a,b-unsaturated and w-unsaturated aliphatic aldehydes. The reaction proceeds in good
yield and provides the target compounds in excellent enantiomeric purity (Scheme 1). For
the route described here benzaldehyde, the enzymes natural substrate, was used.
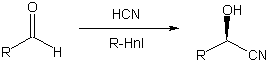
Scheme 1. Synthesis of chiral cyanohydrins under
catalysis of R-Hnl (R=Ph).
Since cyanohydrins are unstable under basic or reductive conditions a
protecting group at the oxygen atom has to be introduced. Both silyl ethers and
tetrahydropyranyl (THP) ethers turned out to be satisfactory protecting groups in the
conversion described here. After protection the nitrile moiety could be reduced with
diisobutylaluminum hydride (DIBAL-H). The aluminum complex originally formed was
decomposed by the addition of acid. The formed O-protected chiral a-hydroxy aldehyde was then used in a Horner-Wittig reaction with
(carbethoxymethyl)- diphenylphosphine oxide, thus forming a chiral g-hydroxy-a,b-unsaturated ester (Scheme
2).
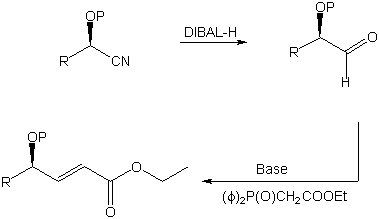
Scheme 2. Synthesis of a g-hydroxy-a,b-unsaturated ester (R=Ph).
This reaction proceeded in a yield of 86% and the product
formed had an enantiomeric purity of 96% as determined by HPLC [6]. NMR spectroscopy
showed that the conversion had proceeded with complete or almost complete E-selectivity
[7]. The results indicate that this is a good and reliable method for the synthesis of
this class of compounds. The reaction also proceeds well starting from aliphatic and w-unsaturated chiral
cyanohydrins (R=propyl or 3-butenyl). The scope of this transformation, as well as its
extension to other Horner-Wittig reagents, is presently under investigation.
References:
1. Zandbergen, P.; Brussee, J.; van der Gen, A; Kruse, C.G.
Tetrahedron: Asymmetry 1992, 3, 769-774.
2. Warmerdam, E.G.J.C.; van der Rijn, R.D.; Brussee, J.; Kruse, C.G., van
der Gen, A. Tetrahedron: Asymmetry 1996, 7, 1723.
3. Jackson, W.R.; Jacobs, H.A.; Jayatilake, G.S.; Matthews, B.R.; Watson,
K.G. Aust. J. Chem. 1990, 43, 2045-2062.
4. Hulsbos, E.; Marcus, J.; Brussee, J.; van der Gen, A. Tetrahedron:
Asymmetry 1997, 8, 1061-1067.
5. Effenberger, F.; Hopf, M.; Ziegler, T.; Hudelmayer, J.; Chem. Ber.
1991, 124, 1651-1659.
6. The enantiomeric purity was determined by HPLC using a Daicel
Chiralcel OD-column (flow = 1 ml/min, l = 254 nm) . As eluent a mixture of hexane and 2-propanol was used (95 / 5).
7. E-selectivity was determined by NMR spectroscopy (Bruker DPX-200
instrument) and was based on the coupling constant of the hydrogen atoms at the newly
formed double bond. The found value of 15 Hz is typical of an E double bond. No
peaks belonging to the Z isomer could by found.
More information about this subject and other projects of our group can be found on our
homepage:
http://wwwchem.leidenuniv.nl/bor/index.htm
You can also reach us by e-mail:
[email protected]