[A0011]
Synthesis of
4,5-dihydroimidazole-2-carboxamides
V.
N. Yarovenko, S. A. Kosarev, I. V. Zavarzin and M. M. Krayushkin
N.D.Zelinsky Institute of Organic
Chemistry, Russian Academy of Sciences, 47 Leninsky prosp., 117913 Moscow, Russian
Federation
E-mail: [email protected]
Received: 14 August 1999 / Uploaded: 21 August 1999
Abstract:
A convenient procedure was developed for the preparation of
4,5-dihydroimidazole-2-carboxamides by the reaction of monothiooxamides with
ethylenediamine.
Keywords: Monothiooxamides, 4,5-dihydroimidazole-2-carboxamides, ethylenediamine, amines.
4,5-Dihydroimidazole derivatives find application as corrosion inhibitorsl, 2 and as starting compounds in the synthesis of biologically active compounds, for example, 4,5-dihydroimidazole-2-carboxamides, possessing anti-hypertensive properties.3
Previously, we have developed a simple approach to the synthesis of monothiooxamides4 by the reactions of chloroacetamides with a solution of elemental sulfur in amines (prepared in advance) at room temperature. In this work, we studied the reactions of monothiooxamides with N-nucleophiles to develop a simple convenient procedure for the preparation of 4,5-dihydroimidazole-2-carboxamides under mild conditions.
It is
known5 that the reactions of primary amines with thioamides can either proceed
with the formation of an imine fragment or result in transamidation. With the aim of
examining general regularities of reactions of monothiooxamides with amines, we first
studied the reactions of N(O)-phenylmonothiooxamides (1) with primary amines.
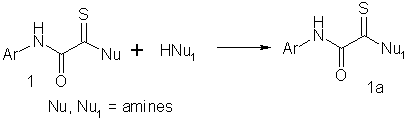
It was
found that transamidation did not affect the C=S group and the amino group of the amide
fragment. The highest yield of compound (1a) was obtained in the reaction with
N(S)-morpholino derivatives of monothiooxamides, which can be obtained from
chloroacetamides in high yields.4 The electron-acceptor and electron-donor
substituents in the benzene ring of the amide fragment have no noticeable effect on
transamidation under the action of primary amines.
With the aim of synthesizing 4,5-dihydroimidazole-2-carboxamides, we studied the reactions of N(S)-morpholino derivatives (1a-c) with ethylenediamine. The reactions were carried out in ethylenediamine or DMF at ~20 °C. 4,5-Dihydroimidazole-2-carboxamides (2a-c) were prepared in 60—90% yields.
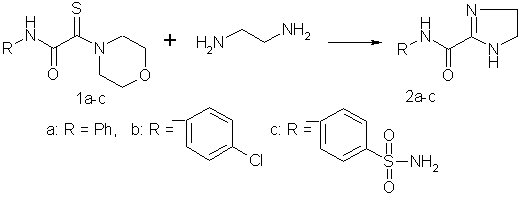
Based on the results of
transamidation of monothiooxamides, it can be concluded that the first stage of the
reaction with ethylenediamine involves the replacement of the morpholine fragment to form
the corresponding monothiooxamide. We examined the possibility of the synthesis of
4,5-dihydroimidazole-2-carboxamides in one stage by the reaction of chloroacetanilides
with a solution (prepared preliminarily) of elemental sulfur in ethylenediamine. It
appeared that the direction of the reaction depended on the order in which the reagents
were introduced into the reaction. When a solution of elemental sulfur in ethylenediamine
was added to chloroacetanilide, bis(thiooxamide) (3) was formed.
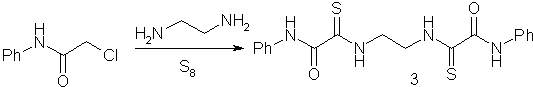
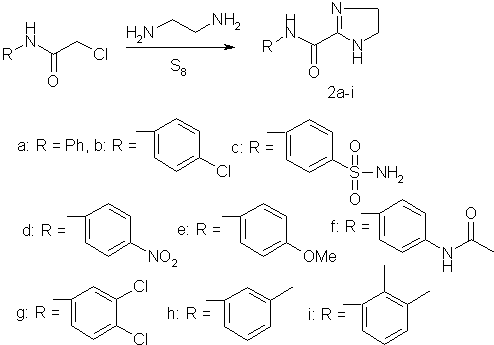
When chloroacetanilide
was added to a solution of sulfur in ethylenediamine, the corresponding
4,5-dihydroimidazole-2-carboxamides (2a-i) were obtained in 50—80% yields.
Thus, we developed a simple procedure for the synthesis of 4,5-dihydroimidazole-2-carboxamides from readily available reagents.
The IR spectra were recorded on a Specord IR-80 spectro-photometer in KBr pellets. The H1 NMR spectra were obtained on Bruker AC-200 (200 MHz) and Bruker WM-250 (250 MHz) instruments in DMSO-d6 relative to HMDS. The mass spectra were measured on a Varian MAT CH-6 instrument with direct inlet of the sample into the ion source with a control voltage of 1.75 kV; the energy of ionizing electrons was 70 eV. The melting points were measured on a Boetius table and are uncorrected. The reaction mixtures were analyzed and the purity of the products isolated was monitored by TLC on Silufol UV-254 plates in an EtOAc—hexane system (1:1, v/v).
Reactions of N(O)-arylmonotbiooxamides with primary amines (general procedure). N(O)-Arylmonothiooxamide (0.10 g) was dissolved in the corresponding amine (1 ml). The solution was kept at room temperature until the initial compound disappeared (TLC control) and then poured into water (20 ml). The precipitate that formed was filtered off, washed with water, dried, and recrystajlized from ethanol.
Reactions of N(O)-arylmonothiooxamides
with ethyienediamine.
N2-phenyl-4,5-dihydro-1H-2-imidazolecarboxamide (2a). A solution of S-morpholino-N(O)-phenylmonothiooxamide (0.10 g, 0.4 mmol) in ethylenediamine (1 ml, 15 mmol) was kept for 1.5 h at room temperature. Then the reaction mixture was poured into water and the precipitate that formed was filtered off and dried. After recrystallization from ethanol, the yield of compound 2a was 0.23 g (60%), m.p. 195-196 °C.
N2-(4-chlorophenyl)-4,5-dihydro-1H-2-imidazolecarboxamide (2b). The reaction with monothiooxamide was carried out analogously. The yield of compound 2b was 0.07 g (90%), m.p. 235—236.5 °C (from ethanol).
N2-[4-(aminosulfonyl)phenyl]-4,5-dihydro-1H-2-imidazolecarboxamide (2c). A solution of monothiooxamide (0.10 g, 0.3 mmol) in ethylenediamine (2.0 ml, 1.79 g, 29 mmol) was kept for 2 h at room temperature (TLC control, EtOAc—hexane, 3 : 1). The reaction solution was poured into water (50 ml) and extracted with EtOAc (3?30 ml). The organic layer was dried with MgSO4 and the solvent was evaporated in vacuo. The residue was crystallized from ethanol. The yield was 0.05 g (63%), m.p. 280 °C.
Reactions of chloroacetamides with
ethyienediamine and elemental sulfur (general procedure for the preparation of
4,5-dihydroimidazole-2-carboxamides). A mixture of sulfur (1-0 g, 30 mmol) and
ethylenediamine (10 mL, 150 mmol) was stirred for 30 min at room temperature until the
dark-red solution was formed. Then chloroacetanilide (6 mmol) was added portionwise with
stirring over 10 min. The reaction mixture was stirred for 30 min and poured into water
(100 mL). The precipitate was filtered off, washed with water, dried, dissolved in
acetone, and filtered to remove unconsumed sulfur. The acetone solution was concentrated
and the residue was recrystallized from ethanol. The characteristics of the resulting
4,5-dihydroimidazole-2-carboxamides 2a-i are given in Table1.
N,N’-Bis(2-anilino-2-oxothioacetyl)-l,2-ethylenediamine (3). A mixture of sulfur (0.5 g, 15.6 mmol) and ethylenediamine (0.2 ml, 0.18 g, 2.9 mmol) was stirred for 30 min at room temperature. Then the reaction mixture was added portionwise to a solution of chloroacetamide (0.5 g, 2.95 mmol) in DMF (5 ml) over 10 min. After 30 min (TLC control), the mixture was poured into water (50 mL). After 12 h, the precipitate that formed was filtered off, dried, dissolved in acetone, and filtered to remove unconsumed sulfur. The acetone solution was concentrated and the residue was recrystallized from ethanol. The yield was 0.40 g (36%), m.p. 198-200 °C. Found (%): C, 56.10;
H, 4.39; N, 14.37. C18H18N4O2S2 . Calculated (%): C, 55.94;
H, 4.69; N, 14.50. H1 NMR, d: 3.98 (m, 4 H. 2 -??-);
7.25 (t, 2 H, 2 H arom.); 7.37 (t, 4 H, 4 H arom.); 7.75 (d, 4 H, 4 H arom.); 10.30 (s, 2 H, 2 NH); 11.00 (s, 2 H, 2 NH). MS, m/z: 386 [M]*
Table 1. Characteristics of the synthesized 4,5-dihydroimidazole-2-carboxamides (2a—i)
1.Jap. Pat. 09/007821; Chem. Abstrs., 1997, 126, 194376?.
2. A. J. Swift, Microchim. Acta, 1995, 120, 149.
3.V. P.Arya, R. S. Grewal, C. L. Kaul, J. David, S. J. Shenoy, and V. Nankan, Ind. J. Chem., 1977, 1513, 148.
4.V. N. Yarovenko, I. V. Zavarzin, A. Yu. Martynkin, and M. M. Krayushkin, 18th Intern. Symp. on the Organic Chemistry of Sulfur, Book of Abstracts, Florence, Italy, 1998, P-106, 104.
5. K. A. Petrov and L. N. Andreev, Usp. Khim., 1971, 40, 1014 [Russ. Chem. Rev., 1971, 40 (Bngl. Transl.)].
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0011 as the message subject of your e-mail.