

[A00012]


Robert Weisa, Klaus Schweigera and Walter M. F. Fabianb aInstitute of Pharmaceutical Chemistry, Schubertstr. 1
Received: 24 July 1999 / Uploaded: 6 August 1999
Depending on the substituent at C-4 (OH or MeNH) 3-acyl-5,6-dihydropyridinones 1 and 2 react with
hydroxylamine to give either isoxazolo[5,4-c]pyridinone 3
and isoxazolo[4,3-c]pyridinone 4, respectively.
The tautomeric equilibria of 1 and 2 are
investigated by means of NMR spectroscopy and density functional
calculations. The mechanisms of formation of 3
and 4 are interpreted with the aid of semiempirical molecular orbital
calculations.
Isoxazolo[4,5-c]pyridinones, e.g. 3, show a wide variety of biological activity. They can act as hypolipidemic agents and are important precursors for hypnotics, muscle relaxants as well as tranquillizers[1]. The isomeric isoxazolo[4,3-c]pyridinones, e.g. 4, are relevant synthons for analogues of herbicides[2]. In the following, the synthesis of the two isomeric compounds 3 and 4, the feasibility to influence the isomer ratio as well as their tautomeric equilibria as evidenced by NMR spectroscopy and densitiy functional calculations, will be described.
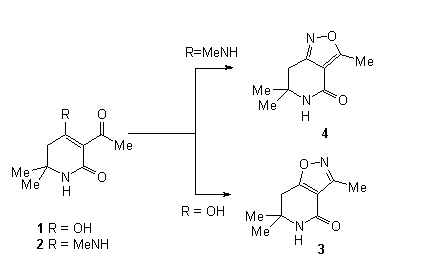
Acylation of 4-methylamino-5,6-dihydropyridin-2(1H)-one under Friedel-Crafts conditions selectively yields the 3-acetyl derivative 2. Alkaline hydrolysis of 2 is used to synthesize the 4-hydroxy derivative 1. In contrast to 3-acetyl-4-hydroxy-2(1H)-quinolinones, which on reaction with hydroxylamine, yield the oximes [4], both 1 as well as 2 cyclize to isoxazolopyridinones. Interestingly, there is a strong dependence of the outcome of these cyclizations on the substituent at C-4: In case of the 4-hydroxy derivative 1 exclusively the isoxazolo[4,5-c]pyridinone 3 is formed. In contrast, reaction of the 4-methylamino derivative 2 with hydroxylamine results in a mixture of 3 and the isomeric isoxazolo-[4,3-c]pyridione 4. The composition of this mixture can be regulated by the reaction conditions, namely the acidity of the solution:
| starting material | basic | neutral | weakly acidic | acidic |
| 1 | decomposition | 3 (52%) | 3 (63%) | decomposition |
| 2 | 4 (15%) | 4(17%) | 3(27%) + 4(24%) | 3 (32%) |
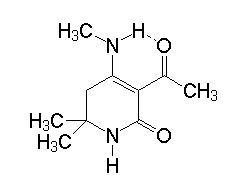
4-Methylaminopyridin-2(1H)-one 2 exists according to NMR spectroscopy in one single tautomeric form with an intramolecular hydrogen bond between the 4-MeN-H and the 3-acetyl group:
In contrast, for 1 both a tautomeric as well as configurational equilibrium was established by NMR spectroscopy:
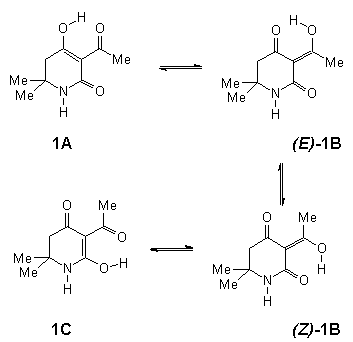
Structure 1C appears to be negligible, 1A and (E)-1B are in
a rapid equilibrium (approx. 20%) and (Z)-1B is the dominant species.
Hybrid Hartree-Fock/densitiy functional calculations (Table 1)
corroborate these experimental results.
In order to rationalize this unexpected behavior and to establish the
mechanisms of these reactions, semiempirical molecular orbital calculations (PM3)
including solvent effects (SCRF approximation) were performed. For 1, both
tautomeric forms - 1A and (Z)-1B - were taken into account; for 2
only the amino tautomer was treated. For each structure four different pathways were
considered:
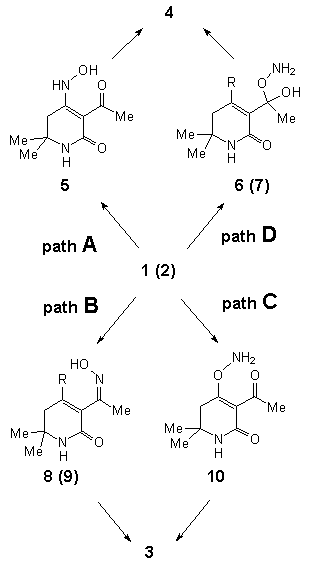
The detailed mechanisms for these four pathways turned out to be quite
complicated [5]; thus, in Table 2 only the
relative energies of the rate determining transition states are collected.
The main conclusions from these calculations are:
[1] J. Nadelson (Sandoz), US 4 049 813 (1977); (1978); CA 88, 68 62w;
J. Nadelson(Sandoz), Ger Offen 2 609 127 (1976); (1976); CA 85, 192582r; J. Nadelson
(Sandoz), US 4 131 679 (1978); (1979); CA 90, 186802z.
[2] P. Roschger and W. Stadlbauer, Liebigs Ann. Chem. 821 (1990); W.
Steinschifter, W. Fiala and W. Stadlbauer, J. Heterocycl. Chem. 31, 1647 (1994); T. Kappe
and B. Schnell, J. Heterocycl. Chem. 33, 663 (1996).
[3] R. Weis, K. Schweiger and W. M. F. Fabian, Monatsh. Chem. 129,
1285 (1998).
[4] T. Kappe, R. Aigner, M. Jobstl, P. Hohengassner and W.
Stadlbauer, Heterocycl. Commun. 1, 341 (1995).
[5] W. M. F. Fabian, K. Schweiger and R. Weis, J. Phys. Org. Chem., in
the press.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0012 as the message subject of your e-mail.