
[A0017]
 |
|
Site and Stereoselectivities in the High Pressure Reaction
of Furan with Dimethyl 7-isopropylidenebicyclo[2.2.1]
hepta-2,5-diene-2,3-dicarboxylate and Tetramethyl
7-isopropylidenebicyclo[2.2.1]
hepta-2,5-diene-2,3,5,6-tetracarboxylate
Ronald N. Warrener*Douglas N. Butler Shudong Wang and Edward R. T. Tiekink
*Professor Ron Warrener.
Director, Centre for Molecular Architecture, Central Queensland University,
Bruce Highway, ROCKHAMPTON QLD 4702
Australia
Fax: 61 7 49 309917, Tel.: 61 7 4930 9845, E-mail: [email protected]
Received: 3 August 1999 / Uploaded: 11 August 1999
|
|
|
|
|
The incorporation of ester substituents at the p -bond position of norbornadiene serves to activate the system as a dienophile in regular electron-demand Diels-Alder reactions.1 The resultant dimethyl norbornadiene-2,3-dicarboxylate 1 is an ambident dienophile where cycloaddition can occur, in principle, at either of two different p-sites. Reaction of 1 with tropone or 9,10-dimethyl anthracene has been reported to occur with exclusive site selectivity at the unsubstituted p-centre.2 In the corresponding dimethyl 7-oxanorbornadiene-2,3-dicarboxylate 2, tropone again forms an adduct by reaction at the unsubstituted p-bond.3 However, when the diene is changed to furan, oxanorbornadiene 2 gives adducts resulting from attack at each of the p-centres.4 The furan reaction was studied subsequently in more detail, including the effect of Lewis acids,5,6 and it is now accepted that adducts derived from attack at the ester-substituted p-bond of 2 are under kinetic control.6 The initially-formed furan adducts readily undergo retro Diels-Alder under the conditions of the reaction, so secondary adducts are formed by attack at the unsubstituted p-bond (thermodynamic control). These results are in accord with FMO prediction and calculated heats of formation.6 More recently, we have studied cycloadditions to the maleimide-fused 7-oxanorbornadienes 3 where exclusive attack occurs at the maleimide p-centre with cyclopentadiene, furan and 2,6-dimethylfuran,7 and more recently with N-Cbz pyrrole.8 Semi-empirical and ab initiocalculations have been used to assess this site selectivity and also transition state energies have been employed to predict the stereoselectivities of these reactions, with excellent precision.7,8
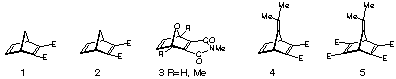
Scheme 1
In the present study, we have examined the cycloaddition reaction of furan 16 with the ester-activated ambident dienophiles 4 and 5. Our objective has been to determine what influence the isopropylidene bridge has on the site selectivity and stereoselectivity when a regular electron-demand Diels-Alder 1,3-diene is involved. Already, it has been reported that dienophile 4 is attacked exclusively at the unsubstituted p-bond when reacted with inverse electron-demand dienes such as s-tetrazine 23 (see later),9 but no other information is available on the cycloaddition reactions of 4 or 5.
Scheme 2
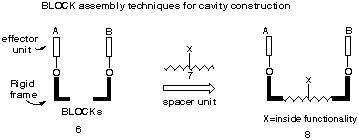
A major driving force for this study is related to our ongoing program directed at the production of inside-functionalised U-type cavity structures10 of type 8, since we wanted to evaluate the potential for the 7-methylene group to act as an inner linking point for attachment of functionality. In this respect the strategy shown in scheme 3 represents an ideal situation in which the syn-facial product 13, formed by selective addition of furan or a N-substituted pyrrole, would yield the turn-frame adduct 15 stereoselectively on treatment with cyclopentadiene. In this way, the exocyclic bridge methylene is centrally located and the R groups attached thereto can be used for the attachment of effector groups.
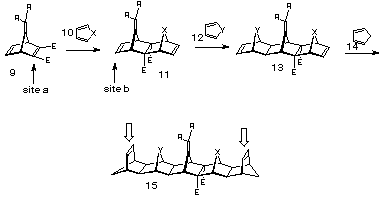
Scheme 3
In practice, no reaction was observed to occur between furan 16 and the ester-activated bicyclic dienophiles 4 or 5 under thermal conditions, even in the presence of Lewis acids. Accordingly, we have conducted the reactions under high-pressure (14 kbar), since this has been reported to promote other furan cycloadditions.11 The results of this study constitute the basis of this paper.
2.1 Reaction of the diester 4 with furan 6.
Reaction of 7-isopropylidene-norbornadiene-2,3-diester 412 with furan 16 under high pressure did proceed at room temperature, but was slow (14 kbar, 40 h, 88%).
Several products were observed in the crude reaction mixture when excess furan was employed, from which one 1:1-adduct (17) and two 2:1-adducts (18 and 20) were separated by chromatography and purified by recrystallisation. The structures of the 2:1-adducts 18 and 20 were solved by X-ray crystallographic methods (Figure 1), while the decision in favour of 1:1-adduct 17 was made on 1H NMR chemical shift and nOe data (vide infra).
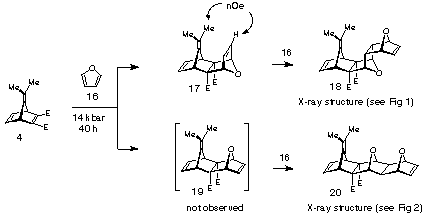
Scheme 4
2.2 Reaction of the tetraester 5 with furan 16.
As no reaction occurred at the unsubstituted p-bond of 4, and forcing conditions promoted preferential cycloaddition at the 7-oxanorbornene p-bond, it was necessary to find some way to promote reaction at this second p-bond if it was going to be useful for the development of cavity systems.
In an attempt to promote reaction at both p-centres of the 7-isopropylidene norbornadiene, activating ester-substituents were introduced onto both the p-bonds (Scheme 5). The required 7-isopropylidene-norbornadiene-2,3,5,6-tetraester 5 (m/z =364) was prepared by reaction of fulvene diester 25 with DMAD 22 at 135-140 o C. The high symmetry of the fulvene tetraester 5 was reflected in the 13 C NMR (7 lines) while the 1 H NMR was even simpler, being comprised of three singlets (d 1.52, C-Me; d 3.81, ester, O-Me; d 4.75, CH).
The requisite fulvene diester 25, used for the synthesis of 5, was itself prepared using a cycloaddition/fragmentation route developed by us earlier and which is outlined in Scheme 3. Site selective attack of the s-tetrazine 23 at the unsubstituted p-bond of the 7-isopropylidene-norbornadiene 4 occurred at room temperature to produce intermediate 24, which fragmented spontaneously to yield 6,6-dimethylfulvene-3,4-diester 25 in 99% yield.9 It is pertinent to note that s-tetrazine 23, an inverse-electron demand diene, reacts at the unsubstituted p-bond of 4, whereas the regular electron-demand dienes react site-selectively at the ester-activated p-bondof 4.
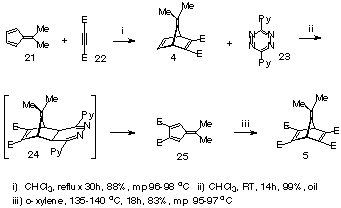
Scheme 5
A similar high-pressure reaction with furan 16 was conducted on the norbornadiene tetraester 5 and while a mixture of 1:1-adducts 26 and 27 and 2:1-adducts 28 and 29 was again produced, no product arising from attack at the alternative p-centre of 5 was observed. This time, two stereoisomeric 1:1-adducts were isolated, together with the corresponding 2:1 products formed by further addition of furan at the 7-oxanorbornene p-bond of the 1:1-adducts (Scheme 6).

Scheme 6
Table 1 1 H NMR chemical shifts for adducts 17, 26 and 27
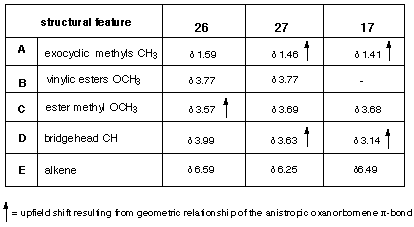
The stereochemistry of the 1:1-adducts 17, 26 and 27 were determined by 1 H NMR chemical shift data, where the anisotropy of the 7-oxanorbornene p-bond played a key role in the structural assignment (see Table 1 and Scheme 6). Fortunately, both isomers in the tetraester series were available so that relative shift data allowed comparisons for several different sets of protons. The methyl groups of the 7-isopropylidene unit were affected by the proximate 7-oxanorbornene p-bond and this caused an upfield shift of 0.13 ppm in 27 relative to isomer 26. A related effect of the proximate 7-oxanorbornene p-bond on the bridgehead protons of the all-carbon bridge (designated Hb in Figure 1) in 27 resulted in a shielding (0.36 ppm) relative to those in isomer 26. In this case molecular modelling (see later) confirms that protons Hb fall within the shielding cone of the p-bond. The upfield shift (0.12 ppm) of the centrally located ester methyl groups in 26 reflect the additional shielding provided by the second p-bond, relative to 27. While these shifts are not large, in combination they provide strong evidence for the assignments.
A comparison between these chemical shift data for 26 and 27, with the 1:1-adduct 17 formed from furan 16 and 4 (Table 2) allowed confirmation of the stereochemistry. The assignment was supported further by the observation of a nOe between the vinyl proton Ha and the methyl substituents of the isopropylidene group.
The structures for the 2:1-adducts 28 and 29 were assigned by comparison between the 1H NMR chemical shifts displayed by the corresponding adducts 18 and 20 formed from the reaction of 16 with 4 for which X-Ray structures have been obtained. These comparisons are tabulated in Table 2 and place the new structures on firm ground.
Table 2
1H NMR data for the 2:1-adducts 18 and 20 (established by X-Ray structure)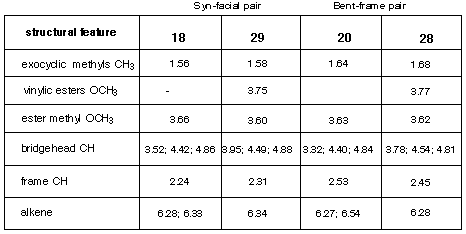
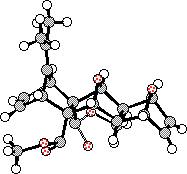
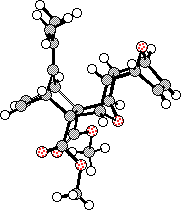
Figure 2. X-Ray structures for bis-adducts 28 and 29.
3. Molecular Modelling and X-Ray Data
Molecular modelling experiments on compounds 18 and 20 were conducted at the AM1 level (Table 4). These results show excellent agreement with the x-ray data for these same compounds. Accordingly, we have confidence in using the AM1 figures when assessing the structures of other key compounds in this series.
Table 3. Crystal data for compound 20 and 18
| Compound | 20 | 18 |
| Formula | C22H24O6 | C22H24O6 |
| Formula weight | 384.4 | 384.4 |
| Crystal system | monoclinic | monoclinic |
| Space group | P21/c | P21/c |
| a (Å) | 14.980(4) | 13.473(2) |
| b (Å) | 14.900(5) | 8.059(4) |
| c (Å) | 8.698(3) | 17.442(2) |
| b (o) | 95.83(2) | 99.420(9) |
| V (Å3) | 1931.3(9) | 1868.2(8) |
| Z | 4 | 4 |
| Crystal size (mm) | 0.08 x 0.13 x 0.29 | 0.32 x 0.32 x 0.48 |
| Dc (g cm-1) | 1.322 | 1.367 |
| F(000) | 816 | 816 |
| m (cm-1) | 0.96 | 0.99 |
| trans. factors | 0.890 - 1 | 0.955 - 1 |
| No. of data collected | 3814 | 3725 |
| ømax (o) | 25.0 | 25.0 |
| No. of unique data | 3570 | 3566 |
| No. of reflections with I ³ 3s(I) | 1459 | 2339 |
| R | 0.052 | 0.035 |
| Rw | 0.045 | 0.035 |
| Residual rmax (e Å-3) | 0.27 | 0.16 |
Table 4. Comparison of experimental and calculated bond length (Å) at AM1 theory.
| parameter | observed |
calculated 20 |
D |
observed (X-ray) |
calculated 18 |
D |
| O15-C1 | 1.438 | 1.454 | 0.016 | 1.436 | 1.458 | 0.022 |
| O15-C8 | 1.430 | 1.450 | 0.020 | 1.436 | 1.454 | 0.018 |
| O18-C10 | 1.443 | 1.464 | 0.021 | 1.442 | 1.471 | 0.029 |
| O18-C13 | 1.437 | 1.466 | 0.029 | 1.445 | 1.472 | 0.028 |
| C1-C2 | 1.568 | 1.564 | 0.040 | 1.563 | 1.572 | 0.009 |
| C1-C14 | 1.523 | 1.545 | 0.022 | 1.528 | 1.534 | 0.006 |
| C2-C3 | 1.570 | 1.567 | 0.003 | 1.582 | 1.555 | 0.027 |
| C2-C7 | 1.586 | 1.608 | 0.022 | 1.586 | 1.613 | 0.027 |
| C3-C4 | 1.526 | 1.532 | 0.008 | 1.523 | 1.537 | 0.014 |
| C3-C16 | 1.515 | 1.530 | 0.015 | 1.512 | 1.532 | 0.020 |
| C4-C5 | 1.319 | 1.354 | 0.035 | 1.313 | 1.354 | 0.041 |
| C5-C6 | 1.520 | 1.528 | 0.008 | 1.526 | 1.535 | 0.009 |
| C6-C7 | 1.593 | 1.563 | 0.030 | 1.568 | 1.558 | 0.010 |
| C6-C16 | 1.520 | 1.533 | 0.013 | 1.523 | 1.532 | 0.009 |
| C7-C8 | 1.546 | 1.565 | 0.019 | 1.570 | 1.566 | 0.004 |
| C8-C9 | 1.535 | 1.543 | 0.008 | 1.520 | 1.536 | 0.016 |
| C9-C10 | 1.553 | 1.553 | 0.000 | 1.562 | 1.555 | 0.007 |
| C9-C14 | 1.563 | 1.585 | 0.022 | 1.545 | 1.587 | 0.042 |
| C10-C11 | 1.520 | 1.535 | 0.015 | 1.502 | 1.529 | 0.027 |
| C11-C12 | 1.318 | 1.353 | 0.035 | 1.314 | 1.354 | 0.040 |
| C12-C13 | 1.513 | 1.535 | 0.022 | 1.504 | 1.529 | 0.025 |
| C13-C14 | 1.543 |
1.553 |
0.010 |
1.564 | 1.555 | 0.009 |
| C17-Me | 1.511 |
1.485 |
0.036 | 1.506 | 1.485 | 0.021 |
| C16-C17 | 1.315 |
1.332 |
0.017 | 1.321 | 1.334 | 0.013 |
| Dimethyl-7-isopropylidenebicyclo[2.2.1]hepta-2,5-dien-2,3-dicarboxylate (4) |
A solution of 6,6-dimethylfulvene (2.00 g, 18.87 mmol) and DMAD (5.36 g, 37.74 mmol) in CHCl3 (15 ml) was heated under reflux for 30 h. The solvent was removed and the resultant yellow-coloured residue slowly solidified. This solid was recrystallised from EtOAc to give a colourless crystalline product (3.59 g, 77%). m.p. 96-98 o C (lit.21190% yield, m.p. 101 o C); 1H nmr (CDCl3) d: 1.48 (6H, s, CH3 x 2), 3.79 (6H, s, OCH3), 4.42 (2H, t, J = 2.3 Hz, H1,4), 7.00 (2H, t, J = 2.3 Hz, H5,6).
The above diester 4 (1.29 g, 5.59 mmol) was treated with furan (5 ml) and compressed at 14 kbar pressure for 40 h. The excess furan was evaporated off under reduced pressure below 30 o C and the resultant colourless residue was chromatographed (silica gel, EtOAc/PE, 3:4, v/v) to provide the 1:1 adduct 17 in the first fractions, followed by the 2:1 exo,exo;exo,exo-adduct 20 and 2:1 exo,endo;exo,endo-adduct 21.
Tetramethyl-7-isopropylidenebicyclo[2.2.1]hepta-2,5-dien-2,3,5,6-tetracarboxylate (5)

A mixture of the above compound 15 (0.58 g, 2.61 mmol) and DMAD (1.56 g, 11.00 mmol) in o-xylene (2 ml) was heated at reflux for 18 h. The solvent was removed. The resultant residue was chromatographed (silica gel, EtOAc/PE, 5:6, v/v) to afford the crude product, which was crystallised from EtOAc as colourless crystals (0.79 g, 83%);
m.p. 95-97 oC (lit.241 m.p. 100 o C, yield 99%);
1H NMR (CDCl3) d: 1.52 (6H, s, CH3x2), 3.81 (12H, s, OCH3x4), 4.75 (2H, s, H1,4);
13 C NMR (CDCl3) d: 18.53 (CH3), 52.33 (OCH3), 56.15 (C1,4), 105.49 (C8), 150.45 (C2,3,5,6), 158.65 (C7), 163.79 (OCO).
Mass spectrum: m/z 364 (M+, 17.3%), 333 (18), 332 (65), 317 (13), 305 (43), 304 (34), 289 (12), 285 (12), 273 (37), 272 (37), 261 (26), 246 (12), 245 (45), 244 (26), 242 (10), 241 (39), 235 (10), 222 (24), 217 (11), 214 (17), 213 (24), 201 (10), 192 (13), 191 (100), 187 (11), 159 (14), 155 (10), 143 (13), 129 (15), 128 (25), 127 (18), 115 (28), 105 (13), 103 (28), 91 (19), 85 (11), 83 (18), 79 (11), 78 (13), 77 (30), 59 (78), 41 (23) ;
The above tetracarboxylate 5 (0.677 g, 1.86 mmol) was treated with furan 16 (4 ml) under 14 kbar for 16 h. The excess furan was evaporated under reduced pressure below 30 o C and the resultant residue was chromatographed (silica gel, EtOAc/PE, 5:2, v/v) to afford the 1:1 adducts in the first fractions, followed by 2:1 exo,exo,exo-adduct and exo,endo, exo-adduct, respectively.
(1a,2a,3b,6b,7a,8a,9b) Dimethyl-11-isopropylidene-13-oxatetracyclo [6.2.1.13,6.02,7]dodeca-4,10-dien-2,7-dicarboxylate (17)
m.p. 176-178 o C;
1H NMR (CDCl3) d: 1.41 (6H, s, CH3 x 2), 3.14 (2H, dd, J = 2.3, 1.8 Hz, H3,6), 3.68 (6H, s, OCH33 x2), 5.07 (2H, t, J = 1.0 Hz, H1,8), 6.22 (2H, t, J = 1.0 Hz, H9,10), 6.49 (2H, dd, J = 2.3, 1.8 Hz, H4,5);
13C NMR (CDCl3) d: 19.38 (CH3), 47.15 (C3,6), 52.17 (OCH3), 69.82 (C2,7), 83.67 (C1,8), 110.01 (C12), 132.38 (C9,10), 139.45 (C4,5), 147.80 (C11), 174.00 (OCO).
Analysis: C18H20O5 requires: C, 68.34; H, 6.31%. Found: C, 68.20; H, 5.82%.
(1b,2b,3a,6a,7b,8b,9a,10a,13a,14a)-Dimethyl-16-isopropylidene-15,18-dioxahexacyclo[6.6.1.13,6.110,13. 02,7.09,3,14]octadeca-4,11-diene-2,7-dicarboxylate (18)
m.p. 179-180 o C;
1H NMR (CDCl3) d: 1.64 (6H, s, CH3x2), 2.53 (2H, dd, J = 1.6, 3.2 Hz, H9,14), 3.32 (2H, t, J = 2.1 Hz , H3,6), 3.63 (6H, s, OCH3), 4.40 (2H, s, H1,8), 4.82 (2H, m, H10,13), 6.27 (2H, m, H11,12), 6.54 (2H, t, J = 2.1 Hz, H4,5);
13 C NMR (CDCl3) d: 20.32 (CH3), 45.33 (C9,14), 46.34 (C3,6), 52.17 (OCH3), 74.57 (C2,7), 79.98 (C10,13), 80.16 (C1,8), 110.17 (C17), 134.52(C11,12), 140.33 (C4,5), 146.04 (C16), 173.52 (OCO).
Analysis: Calc. for C22H24O6 requires: C, 68.74; H, 6.29. Found: C, 68.57; H, 6.19.
(1a,2b,3a,6a,7b,8a,9b,10a,13a,14b)-Dimethyl-16-isopropylidene-15,18-dioxahexacyclo[6.6.1.13,6.110,13. 02,7.09,14]octadeca-4,11-diene-2,7-dicarboxylate (20)
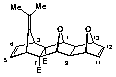
m.p. 173-174 o C;
1H NMR (CDCl3) d: 1.56 (6H, s, CH3 x2), 2.24 (2H, s, H9,14), 3.52 (2H, t, J = 1.8 Hz, H3,6), 3.66 (6H, s, OCH3 x2), 4.42 (2H, s, H1,8), 4.86 (2H, m, H10,13), 6.28 (2H, t, J = 1.8 Hz, H4,5), 6.33 (2H, m, H11,12);
13C NMR (CDCl3) d: 19.80 (CH3), 46.50 (C9,14), 51.60 (C3,6), 51.68 (OCH3), 70.01 (C2,7), 80.78 (C10,13), 84.24 (C1,8), 107.64 (C17), 137.17 (C11,12), 137.93 (C4,5), 143.29 (C16), 170.93 (OCO).
Mass spectrum: m/z 384 (M+, 0.8%), 1.7 (18), 106 (100), 91 (47), 77 (10.6), 68 (20);
Analysis: C22H24O6 requires: C, 68.74; H, 6.29. Found: C, 68.37; H, 6.19.
Dimethyl-6,6-dimethylfulvene-3,4-dicarboxylate (25)
![]()
A solution of dicarboxylate 4 (1.04 g, 4.81 mmol) and s-tetrazine (1.02 g, 4.32 mmol) in CHCl3 (6 ml) was stirred at room temperature for 14 h. The solution was diluted with CHCl3. The organic layer was washed with aqueous HCl (50%), water, dried and the solvent wasremoved to produce a yellow gum in quantitative yield (lit.241 yield 99%).
1H NMR (CDCl3) d: 2.31 (6H, s, CH3x2), 3.82 (6H, s, OCH3), 7.14 (2H, s, H2,5);
13 C NMR (CDCl3) d: 23.95 (CH3), 51.70 (OCH3), 127.44 (C2,5), 133.56 (C6), 139.57 (C1), 164.00 (C4,5), 165.10 (OCO).
(1a,2b,3a,6a,7b,8a,9a)Tetramethyl-12-isopropylidene-11-oxatetracyclo[6.2.1.13,6.02,7]dodeca-4,9-dien-2,7,4,5-tetracarboxylate (26)
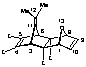
1:1 Adduct 26 (minor product):
m.p. 175-178 o C;
1H NMR (CDCl3): 1.59 (6H, s, CH3x2), 3.57 (6H, s, OCH3x2), 3.77 (6H, s, OCH3x2), 3.99 (2H, s, H3,6), 4.86 (2H, t, J = 0.9 Hz, H1,8), 6.59 (2H, t, J = 0.9 Hz, H9,10);
13 C NMR (CDCl3) d: 19.63 (CH3), 51.87 (OCH3), 51.90 (OCH3), 53.84 (C3,6), 70.53 (C2,7), 84.23 (C1,8), 111.61 (C13), 138.02 (9,10), 143.00 (C4,5), 146.56 (C12), 164.17 (OCO), 170.80 (OCO).
(1a,2a,3b,6b,7a,8a,9b) Tetramethyl-12-isopropylidene-11-oxatetracyclo [6.2.1.13,6.02,7]dodeca-4,9-dien-2,7,4,5-tetracarboxylate (27)
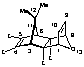
1:1 Adduct 27(major product):
m.p. 176-178 o C;
1H NMR (CDCl3) d: 1.46 (6H, s, CH3x2), 3.63 (2H, s, H3,6), 3.69 (6H, s, OCH3x2), 3.77 (6H, s, OCH3x2), 5.23 (2H, t, J = 0.9 Hz, H1,8), 6.25 (2H, t, J = 0.9 Hz, H9,10);
13C NMR (CDCl3) d: 19.40 (CH3x2), 50.79 (C3,6), 51.97 (OCH3), 52.58 (OCH3), 68.52 (C2,7), 83.98 (C1,8), 113.84 (C13), 134.42 (C9,10), 144.33 (C4,5), 147.33 (C12), 164.09 (OCO), 172.93 (OCO).
Analysis: Calc. for C22H24O9 requires: C, 61.11; H, 5.59. Found: C, 60.91; H, 5.35.
(1a,2b,3a,6a,7b,8a,9b,10a,13a,14b)-Tetramethyl-16-isopropylidene-15,18-dioxahexacyclo[6.6.1.13,6. 110,13.02,7.09,14]octadeca-4,11-dien-2,7,4,5-tetracarboxylate (28)
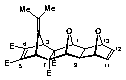
m.p. 173 oC (dec.);
1 H NMR (CDCl3) d: 1.58 (6H, s,CH3x2), 2.31 (2H, s, H9,14), 3.60 (6H, s, OCH3x2), 3.75 (6H, s, OCH3x2), 3.95 (2H, s, H3,6), 4.49 (2 H, s, H1,8), 4.88 (2H, m, H10,13), 6.34 (2H, m, H11,12);
13C NMR (CDCl3) d: 19.72 (CH3), 46.31 (C9,14), 51.97 (OCH3), 51.82 (OCH3), 54.49 (C3,6), 69.30 (C2,7), 80.80 (C10,13), 84.54 (C1,8), 111.25 (C17), 137.16 (C11,12), 141.70 (C16), 145.47 (C4,5), 164.09 (OCO), 169.84 (OCO).
Mass spectrum m/z 500 (M+, 1.4%), 223 (14), 222 (84), 192 (14), 191 (100), 77 (12), 68 (39), 66 (10), 41 (16);
Analysis: Calc. for C26H28O10.0.25H2O requires: C, 61.84; H, 5.69. Found: C, 61.70; H, 5.24.
(1b,2b,3a,6a,7b,8b,9a,10a,13a,14a)-Tetramethyl-16-isopropylidene-15,18-dioxahexacyclo[6.6.1.13,6. 110,13.02,7.09,14]octadeca-4,11-dien-2,7,4,5-tetracarboxylate (29)
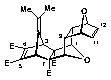
m.p. 199-200 oC;
1 H NMR (CDCl3) d:1.68 (6H, s, CH3x2), 2.45 (2H, dd, J = 1.6, 3.1 Hz, H9,14 ), 3.62 (6H, s, OCH3x2), 3.77 (6H, s, OCH3x2), 3.78 (2H, s, H3,6), 4.54 (2H, s, H1,8), 4.81 (2H, br, H10,13), 6.28 (2H, m, H11,12);
13C NMR (CDCl3) d: 20.32 (CH3), 45.45 (C9,14), 49.86 (C3,6), 52.00 (OCH3), 52.53 (OCH3), 73.46 (C2,7), 79.95 (C10,13), 80.43 (C1,8), 114.12 (C17), 134.62 (C11,12), 144.57 (C4,5), 147.96 (C16), 163.99 (OCO), 172.53 (OCO).
Analysis: Calc. for C26H28O10 requires: C, 62.39; H, 5.64. Found: C, 62.35; H, 5.26.
S.W. thanks the Centre for Molecular Architecture for the award of a CMA PhD Scholarship and the CQU for a fee waiver.
1.Warrener, R.N.; Anderson, C.M.; McCay, I.W.; Paddon-Row, M.N., Aust. J. Chem., 1977, 30, 1481.
2. Pfaendler, H.R.: Tanida, H.; Haselbach, E. Helv. Chim. Acta, 1974, 51, 383.
3. Sasaki, T.; Kanematsu, K.; Hayakawa, K. J. Chem. Soc, Perkin Trans 1, 1972, 1951.
4. Diels, O.; Olsen, S. J. Prakt. Chem., 1940, 156, 285.
5. McCulloch, A.W.; Smith, D.G.; McInnes, A.G., Can. J. Chem. 1973, 51, 4125.
6. McCulloch, A.W.; Smith, D.G.; McInnes, A.G., Can. J. Chem. 1974, 52, 1013.
7. Warrener, R.N.; Elsey, G.M.; Maksimovic, K.; Johnston, M.R.; Kennard, C.H.L., Tetrahedron Lett., 1995, 36, 7753.
8. Sun, G.; Butler, D.N.; Warrener, R.N.; Margetic, D.; Malpass, J.R., Electronic Conference on Heterocyclic Chemistry, 1998.
9. Wilson, W.S.; Warrener, R.N., J. Chem. Soc., Chem. Commun. 1972, 211.
10. a)Warrener, R. N.; Wang, S.; Russell, R. A., Tetrahedron 1997, 53, 3975-3990. b) Warrener, R. N.; Wang, S.; Maksimovic, L.; Tepperman, P. M.; Butler, D. N., Tetrahedron Letters, 1995, 36, 6141-6144. c) Warrener, R. N.; Maksimovic, L.; Butler, D. N., J. Chem. Soc., Chem. Commun., 1994, 1831-1832. d) Warrener, R. N. Chem. in Aust. 1992, 59, 578-581.
11. Isaacs, N. S., Tetrahedron 1991, 47, 8463-8497.
12. Prinzbach, H.; River, J., Helv. Chim. Acta, 1970, 53, 2201-2219.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0017 as the message subject of your e-mail.