
[A0020]
- N-METHYLTYRAMINE AND N,O-DIMETHYLTYRAMINE
Received: 30 July 1999 / Uploaded: 13 August 1999
Keywords: galanthamine synthesis half-products, N-methyltyramine, N-methyltyramine ethers, N,O-dimethyltyramine, arylthioacetamide, Willgerodt-Kindler reaction
N-Methyltyramine and its alkyl or benzyl ethers are common intermediate products in the syntheses of narwedine, and galanthamine alkaloids [1-8]. They are usually produced from the corresponding arylacetamides by lithium aluminium hydride reduction. In order to avoid application of inflammable lithium aluminium hydride bulgarian scientists have transformed arylacetamide to arylthioacetamide with phosphorus (V) sulfide with following reduction of arylthioacetamide with complex sodium borohydride - nickel or cobalt chloride agents [7, 8].
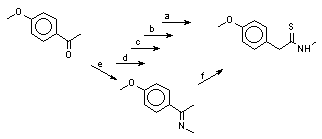
Here we present a new short way route to N-methyltyramine and its ethers via the corresponding aryl-N-methylthioacetamides, shown at the scheme 1 at the example of N-methyltyramine and N,O-dimethyltyramine synthesis.
Scheme 1.

92% 57-58% 91% 98%
total 46-47% total 48-49%
a) H3CCOBr, AlCl3, ClCH2CH2Cl.
b) H3CNH2.HCl, S8 , H3CCO2Na, (H3C)2NCHO, 115oC, 3h.
c) Zn (dust) and 36% HCl(aq.) in ethanol
d) 36% HCl(aq.), refluxion
Anisole was smoothly acetylated in a classical conditions [9] with acetyl bromide and aluminium chloride in dichloroethane to produce 4-methoxyacetophenone in 92% yield.
Phenylacetic acid thioamides are usually synthesized by the Willgerodt-Kindler reaction from the corresponding substituted acetophenones, amines, and sulfur. We have studied several alternative routes to the 4-methoxyphenyl-N-methylthioacetamide synthesis shown at the scheme 2.
Scheme 2.
a) H3CNH2, S8, 170-180oC, 3h (yield 10,4%, and 21,9% by-product amide).
b) H3CNH2.HCl, S8 , H3CCO2Na, (H3C)2NCHO, 115oC, 3h (yield 58,4%).
c) H3CNH2.HCl, S8, N(C2H5)3, 4-H3CC6H4SO3H.H2O, (H3C)2NCHO, 75oC, 72h (yield 57%)
d) H3CNHCHO, S8, 190oC, 20h (yield 35%).
e) H3CNH2 .HCl, Na2CO3, CaO, N(C2H5)3, -7oC (isolated mixture imine : ketone 77:23 (1H NMR) formed, used for the next stage without separation).
f) S8, N(C2H5)3, (H3C)2NCHO, 70oC (total yield on two stages 21,5%).
The best results were produced according to routes b and c.
The key thioamide was isolated chromatographically on alumina or silica gel.
In classical conditions of the Willgerodt-Kindler reaction in the sealed tube [10] the desired thioamide was obtained in only 10.4% yield, while the corresponding by-product amide was also isolated in 21.9% yield. In order to avoid the application of high pressure, two-stage synthesis was carried out via intermediate 4-methoxyacetophenone methylimine [11] by method [12, 13] in 21.5% total yield.
Earlier proposed method of carrying out the Willgerodt-Kindler reaction with volatile lower secondary amines, e. g. dimethylamine, using its hydrochloride and sodium acetate in dimethyl formamide at 100-110oC and atmospheric pressure was developed [14].
We have used a similar method for lower primary amine. Thus methylamine hydrochloride, 4-methoxyacetophenone, sulfur, and a base in dimethyl formamide at 60-110oC and atmospheric pressure gave the desired thioamide in 57-58% yield.
An other route of 4-methoxyphenyl-N-methylthioacetamide synthesis on heating the mixture of 4-methoxyacetophenone, N-methylformamide and sulfur at 170-180oC at atmospheric pressure according to the method [15] 35% yield was achieved.
4-Methoxyphenyl-N-methylthioacetamide is a new compound, forming colorless crystals, m. p. 89-91oC (from ethanol or from diethyl ether) [16].
We have elaborated for the thioamide obtained a new simple and efficient method of reduction with zinc dust and hydrochloric acid in ethanolic solution. N,O-Dimethyltyramine was obtained in 91% yield, which was converted to its hydrochloride, m.p. 179-181oC (from ethanol), (lit. m.p. 181-182oC [17]).
O-Demethylation of N,O-dimethyltyramine to N-methyltyramine was carried out according to earlier described method for N-acetylated compound [18] by refluxing with concentrated hydrochloric acid in the oil bath heated to 170-175oC. The yield was 98%, m. p. 146-149oC (from ethanol with a small amount of hydrochloric acid), lit. m. p. 148.5oC [18].
The total yield of N-methyltyramine was 46-47% on four-stage synthesis and the total yield of N,O-dimethyltyramine was 48-49% in three-stage synthesis starting from anisole.
References
[1] T. Kametani, K. Yamaki, H. Yagi, K. Fukumoto, J. Chem. Soc. (D), 1969, (8), 425-426.
[2] T. Kametani, K. Yamaki, H. Yagi, K. Fukumoto, J. Chem. Soc. (C), 1969, (18), 2602-2605.
[3] T. Kametani (Grelan Pharmaceutical Co., Ltd.) JP 47-13919 (C07D, A61K), 26.04.1972, appl. 42-18662,12.03.1969 (C. A. 1972, 77(7), 48690s).
[4] T. Kametani, K. Shishido, E. Hayashi, C. Seino, T. Kohno, S. Shibuya, K. Fukumoto,
J. Org. Chem, 1971, 36(9), 1295-1297.
[5] T. Kametani (Grelan Pharmaceutical Co., Ltd.) JP 47-46079 (C07D, A61K), 20.11.1972, appl. 45-93228, 23.10.1970 (C. A. 1973, 78,(17), 111577y).
[6] T. Kametani, K. Yamaki, T. Terui, S. Shibuya, K. Fukumoto, J. Chem. Soc. Perkin Trans. I, 1972, (12), 1513-1516.
[7] R. Vlakhov, D. Krikorian, M. Zagorova, M. Chinova, S. Parushev, G. Snatzke, H. J. Shaefer, 11th IUPAC Int. Symp. Chem. Nat. Prod., Symp. Pap. 1978, vol.4, part 2, 251-273 (Ed. N. Marekov, I.Ognyanov, A. Orahovats. Izd. BAN: Sofia, Bulgaria).
[8] R. Vlakhov, D. Krikorian, G. Spassov, M. Khinova, I. Vlakhov, S. Parushev, G. Snatzke, L. Ernst, K. Kieslich, W.-R. Abraham, W. S. Sheldrick, Tetrahedron, 1989, 45(11), 3329- 3345.
[9] Organikum (Organisch-chemisches Grundpraktikum), 15 Auflage, Berlin, 1976. Russisch Auflage: Moskau, 1979, S. 422-423.
[10] K. Kindler, DRP 405675 (12o), 30.10.1924, 17.07.1924; K.85209, 13.03.1923.
(P. Friedlaender, Fortschritte der Teerfarbenfabrikation und verwandter Industrielzweige. Berlin, 1926, 14 Teil, S. 372.).
[11] J. Bjorgo, D. R. Boyd, C. G. Watson, J. Chem. Soc. Perkin Trans. II, 1974, (7), 757-762.
[12] F. Asinger, A. Saus, H. Offermans, F. A. Dagga, Justus Liebigs Ann. Chem., 1969, 72, 119-128.
[13] K. Kindler, W. Peschke, Arch. Pharm. und Ber. Deutsch. Pharm. Ges., 1932, 270 (42 Ber.), (6), 340-353.
[14] J.O.Amupitan, Synthesis, 1983, (9), 730.
[15] R. Wegler, E. Kuhle, W. Schaefer, Angew. Chem., 1958, 70, (11-12), 351-367.
[16] Elemental analysis, %: found: C 61.87; H 6.99; N 7.00; for C10H13NOS, M=195.28, calculated: C 61.51, H 6.71, N 7.17.
MS (m/z), (%): (M+2)+ 197 (46.1), (M+1)+ 196 (60.4), M+ 195 (96.4), M++ 97.5 (46.3), 180 (30.9), 164 (65.5), 149 (41.9), 147 (40.0), 140 (42.0), 121 (100), 107 (61.8), 77 (51.9).
1H-NMR (CCl4-CDCl3), (p.p.m.): 3.05 and 3.09 (2s., 3H, CH3N, S-cis- and S-trans- forms), 3.78 (s., 3H, CH3O), 3.97 (s., 2H, CH2), 6.81 (d., 2H) and 7.12 (d., 2H) (para-C6H4).
UV (ethanol), (nm) (extinction): 210 (11420), 225(sh.); 266 (10190).
IR (nujol), (cm-1): 3210, 1600, 1520, 1505, 1300, 1230, 1185, 1175, 1060, 1020, 825, 810, 760, 600, 540, 510.
[17]. F. B. La Forge, W. F. Barthel, J. Org. Chem. 1944, 9(3), 250-253.
[18] G. S. Walpole, J. Chem. Soc. 1910, 97, 944-947.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0020 as the message subject of your e-mail.