
MICROWAVE ENHANCED HYDROAMINATION OF STYRENE

[a0022]
 |
MICROWAVE ENHANCED HYDROAMINATION OF STYRENE |
 |
Julio A. Seijas*, M. Pilar Vázquez Tato* and M.Montserrat Martínez
Received: 5 August 1999 / Uploaded: 22 August 1999
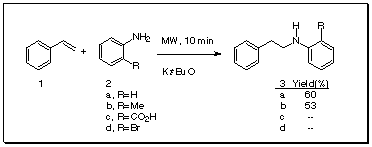
Synthesis of ß-phenethylamines has been a matter of interest due to their pharmacological properties, and there are several methods to prepare ß-phenethylamines. Our group has been trying to develop new methods for the hydroamination of styrene, and recently we have communicated our results1.
But, when we tried to react lithium anilide(PhNH2/BuLi) with styrene in THF at O°C it failed. In a previous work2 there has been reported the hydroamination of styrene with aniline sodium salt by heating for 7 hours at 184°C, and recently, it was reported3 that aniline added to styrene by heating in a sealed tube with Kt-BuO. Now, we attempted to perform the reaction in the absence of solvent and in an open vessel under microwave heating. We used the potassium tert-butoxide as base in a ratio to aniline 1:1, but we have problems with ignition of the reaction mixture. We investigated the influence of several ratios PhNH2/Kt-BuO, founding that a 10% ratio gave the best yield with a 60% of N-phenyl-ß-phenethylamine, isolated by column chromatography.
We carried out, as well, the reaction with 2-methylaniline, affording the addition product in 53% yield. Another anilines employed were 2-bromo and 2-carboxy (antharanilic acid), but both failed in giving addition products, with anthranilic acid we obtained aniline as decarboxylation product.
In conclusion here we present for first time the application of microwaves to hydroamination of styrene. We think it constitutes a good alternative to the sealed tube previous reports, and it opens a new application to microwave heating. We are studying if this methodology can be applied with another amines and to styrene derivatives in general.
ACKNOWLEDGMENTS
We thank Dirección General de Enseñanza Superior for its financial support (PB96-0932)
1.- Seijas, J. A., Vázquez-Tato, M. P., Entenza, C., Martínez, M. M., Ónega, M. G., Veiga, S., Tetrahedron Lett., 1998, 39, 5073.
2.-Wegler, R and Pieper G., Chem. Ber. 1950, 83, 1.
3.-Beller, M., Briendl, C., Riermeier, T. H., Eichberger, M. and Trauthwein, H., Angew. Chem. Int. Ed. Eng., 1998, 37, 3389.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0022 as the message subject of your e-mail.