
an expeditious method to prepare 3-benzylidene-1,3-dihydroindol-2-ones

[a0024]
 |
MICROWAVE ENHANCED KNOEVENAGEL REACTION:
an expeditious method to prepare 3-benzylidene-1,3-dihydroindol-2-ones |
 |
Julio A. Seijas*, M. Pilar Vázquez Tato*, M. Carmen Fernández and Nazaret Arias
Received: 5 August 1999 / Uploaded: 22 August 1999
Knoevenagel reaction has been object of a complete study1, recently the enhancement of this reaction by microwave heating has been reported by several groups2. We became interested in 3-benzylidene-1,3-dihydroindol-2-ones, as they have been reported as natural products from Isatis indigotica3, although they have been known as pharmacological agents from several years4.
We began our studies with conventional refluxing of oxindole in MeOH
with piperidine and the adequate aldehyde.5 In some
examples it took several hours refluxing, thus in order to speed reaction times, we think
in use microwave heating of the reaction mixture in absence of the solvent. We used a
domestic microwave oven, and the reactions were carried in a glass test tube. We found
that reaction times were shorter around 7-11 minutes and yields were almost quantitative.
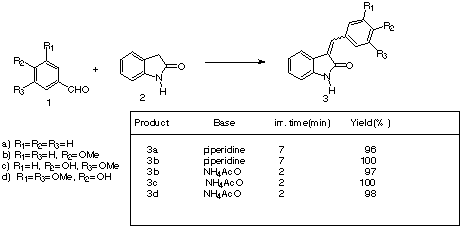 |
An interesting finding was that when we tried to obtain phenol derivatives, microwave irradiation lead to complex reaction mixture. We had to look for an alternative base to replace piperidine. Ammonium acetate gave nice results; yield remained almost quantitative, reaction times were reduced to around 2 minutes, and we could avoid the use of liquid and volatile piperidine. Thus we think that our procedure constitutes a good alternative to conventional and microwave enhanced methods, being an environmentally clean and experimentally simple synthesis.
ACKNOWLEDGMENTS
We thank Dirección General de Enseñanza Superior (DGES) for its financial support (PB96-0932).
1.- G. Jones, Organic Reactions, 1967, 15, 204.
2.- D. Villemin, B. Martin, Synthetic. Commun.,1998, 28, 3201. D. Bogdal, J. Chem. Research (S), 1998, 468.
3.- X. Wu, Y. Liu, W. Sheng, J. Sun, G. Qin, Planta Medica, 1997, 63, 55.
4.- G. L. Baut, M. R. Norrisson, J. C. R. Faverie, J.C. B. Espard, D. H. Caignard, P. Renard, G. Adam, US. Pat. 5382593, 1995.
5.- A. Andreani, M. Rambaldi, A. Locatelli, R. Bossa, I. Galatulas,Eur. J. Med. Chem., 1990, 25, 187.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with a0024 as the message subject of your e-mail.