 |
HYDROAMINATION OF CINNAMYL ALCOHOL |
 |
[a0025]
 |
HYDROAMINATION OF CINNAMYL ALCOHOL |
 |
Julio A. Seijas*, M. Pilar Vázquez-Tato*, Luis Barreiro-Castro and Raquel Romero-Van-der-Schoot
Received: 5 August 1999 / Uploaded: 22 August 1999
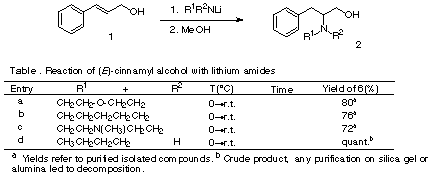
In our group, we have been studying the hydroamination of styrene and its derivatives1. Here, we want to present a preliminary communication about the hydroamination of cinnamyl alcohol. This addition would afford 2-amino-3-phenylpropanols (2), which are of biological interest due to its presence as constituents in the structure of different natural products isolated from many different sources, such as: Allangium lamarckii, Anaphalis subumbellata, Aspergillus flaviceps, A. janus, A. glaucus, Caranthus pusillus, Cystoceria corniculata, Emericellopsis salmosynnemata, Euphorbia fischeriana, Hybanthus enneasperma, Medicago polymorpha, Penicillum canadiense, P.brevicompactum, P. megasporum, Piper aurantiacum and Schismatomma hypothallium.
To our knowledge, there was no previous report of hydroamination on
cinnamyl alcohol, although there has been reported2
addition of alkyllithiums to 1. Thus, lithium amides were prepared by addition of
n-butyllithium to the parent amine at 0°C, and then cinnamyl alcohol dissolved in dry THF
was added drop wise to the lithium amide. We prepared the ß-phenylethylamines shown in
the table. We observed that cyclic secondary amines and aliphatic primary amines gave good
yields of amination, but in other cases: diethyl amine, benzylamine and
N-methylbenzylamine yields were very low. These results agrees with our previous results
for styrene with methyl group in ß position1.
 |
Actually we are trying to get a wider evidence of the behavior of other amines. We are studying as well, the possibility of getting choral induction in these additions.
ACKNOWLEDGMENTS
We thank Dirección General de Enseñanza Superior (DGES) for its financial support (PB96-0932).
1.- Seijas, J. A., Vázquez-Tato, M. P., Entenza, C., Martínez, M. M., Ónega, M. G., Veiga, S., Tetrahedron Lett., 1998, 39, 5073.
2.- Klein, S., Marek, I., Poisson, J-F., Normant, J. F., J. Am. Chem. Soc., 1995, 58, 8853.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0025 as the message subject of your e-mail.