[A0028]
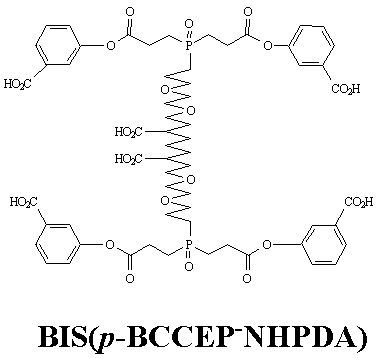
Dendrimeric Organic Reagents for Macromolecular Cross-Linking: Synthetic Approaches to 2,6-Bis[3,6-dioxa-9,9'-bis[bis[2-((p-carboxy phenoxy)carbonyl) ethyl]phosphinyl]nonyl]heptanedioic Acid [Bis(p-BCCEP-NHPDA)]]
Timothy A. Roach and Ramachandra, S. Hosmane*
Laboratory for Drug Design and Synthesis, Department of Chemistry &
Biochemistry
University of Maryland, Baltimore County (UMBC), 1000 Hilltop Circle
Baltimore, Maryland 21250, USA
E-mail: [email protected]
Received: 17 August 1999 / Uploaded: 21 August 1999
Keywords: Dendrimeric polyfunctional Organic Reagents; Cross-Linking Proteins; Hemoglobin; Artificial Blood
INTRODUCTION
Efforts to develop alternatives to blood as an oxygen carrier date back well over half a century with increased efforts in recent years being partly motivated by fears of potential contamination with the viruses responsible for AIDS and hepatitis (1-4). An additional impetus rests in the recognition that wide availability of properly banked blood is absent in many parts of the world. An efficient acellular oxygen carrier could have several distinct advantages over intact red blood cells, including elimination of the need for typing and cross-matching before transfusion and a useful shelf-life greater than the current six weeks or less for packed red blood cells (1). While a number of other alternatives have been explored over the years (1-4), the use of cell-free hemoglobins for an oxygen-carrying resuscitation fluid has excellent prospects since (a) hemoglobin solutions are completely metabolizable and are well tolerated by the body, and (b) hemoglobin is available in virtually unlimited amounts and is relatively inexpensive, (c) hemoglobin is fully saturated with oxygen under ambient conditions, has oncotic activity, and exhibits cooperative oxygen binding behavior. However, there are two major problems associated with using cell-free hemoglobins for transfusions. First, the retention time of cell-free hemoglobins in circulation after infusion is very short (4), and most of the infused hemoglobin is rapidly filtered and eliminated by kidneys in 1-4 hours; second, cell-free hemoglobins have too high oxygen affinity that prevents them from adequately unloading the oxygen acquired from lungs to tissues. Both of these problems have their roots in 2,3-diphosphoglycerate (DPG) and other polyanionic species which are conspicuously absent in cell-free hemoglobins, but are known to be essential co-factors in intact red blood cells. Efforts have since been focused on covalently cross-linking hemoglobin between the two like subunits, e.g., alpha-1 to alpha-2 or beta-1 to beta-2. Such a cross-link is believed to substitute for the native allosteric modifier DPG to lower the oxygen affinity, while at the same time preventing dissociation of the tetramer (1).
Several bifunctional and polyfunctional organic reagents (BORs and PORs)
have been employed in the past to carry out this task (5-12). Varying degrees of success
have been achieved with a number of these reagents, and some of the modified hemoglobin
products are currently undergoing clinical trials (1a,1b). The current thrust in
hemoglobin based blood substitutes is to significantly increase the intravascular
retention time from the current level of several hours to the optimally desired several
days. To this end, our present focus is on design and synthesis of novel dendrimeric
organic reagents (DORs) that are capable of simultaneously forming specific intra-
as well as intermolecular cross-links in cell-free human hemoglobins. We report here our
progress and current status on the synthesis of the title DOR, [Bis(p-BCCEP-NHPDA]

RESULTS AND DISCUSSION
(A) Design of the Title Dendrimeric Organic Reagent (DOR) by
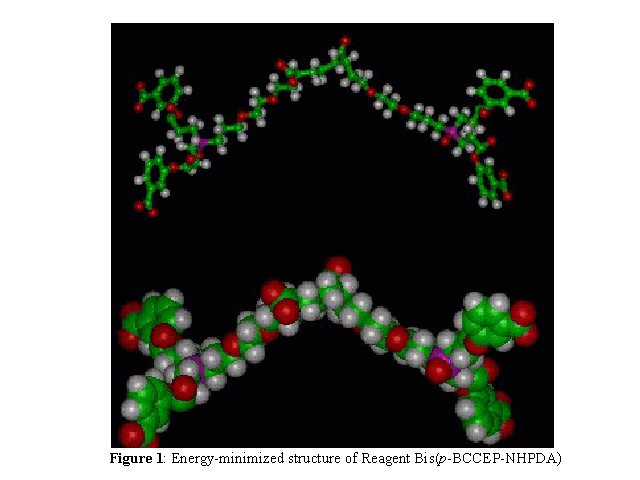
Molecular Modeling: The title DOR was designed employing molecular modeling
techniques. Molecular modeling was performed on a Silicon Graphics workstation, using the
software INSIGHT/DISCOVER (Molecular Simulations, Inc., San Diego, California). The X-ray
coordinates of human OxyHbA0 (13), imported from the Brookhaven National
Laboratory, Upton, New York, were employed for molecular modeling. The reagent was
energy-minimized (see Figure 1), and the two cross-linking sites on each end of the
bifurcate reagent were docked into the two -clefts (DPG pockets) of two independent
hemoglobin tetramers, followed by energy minimization of the reagent-Hb complex. All atoms
that were 15 Å or farther from the ligand were fixed with a temperature constant of 300
K. No constraints were applied to the remaining residues in and around the ligand site.
The complex was minimized to convergence using consecutive Steepest Descent and Conjugate
Gradient (VAO9A) minimization protocols (see Figures 2 and 3). There were no
morse or cross terms.


Progrss Toward Synthesis of the Title Reagent Bis(p-BCCEP-NHPDA):
The synthetic route for the title reagent is outlined in Scheme 1. The synthesis
commenced with di-tert-butyl malonate (1) (Aldrich) which was condensed with
1,2-bis(2-iodoethoxy)ethane (or 1,8-

diiodo-3,6-dioxaoctane) (Aldrich) to obtain [2,2'-(3,6-dioxaoctanediyl)dimalonic acid
tetra-tert-butyl ester)] [bis(t-butyl malonate)-DOD] (3). It is to be
noted that the corresponding tetraethyl ester of 3 (Beilstein Registry No. 1809812;
CAS Reg. No. 78649-90-8) and a series of its homologues are known in the literature. Two
molecules of Compound 3 were further condensed with 1,3-dibromopropane to obtain 5.
Compound 5 contains a central tether that has a middle three-carbon unit joined by
two DOD fragments. This is where the synthesis stands at the present time. Reaction of 5
with two molecules of 6 (which will be prepared from the cross-linking reagent BCCEP
whose synthesis and hemoglobin cross-linking studies we reported recently) (14) will yield
7. The removal of the protecting groups of the latter by treatment with
trifluoroacetic acid in methylene chloride is anticipated to be accompanied by
decarboxylation (of six CO2 molecules) to afford the title Reagent Bis(p-BCCEP-NHPDA).
ACKNOWLEDGMENT
This work was supported by a grant (# RO1 HL48632) from the National Institutes of
Health.
REFERENCES
1). (a) Ritter, S. K., Passing a Blood Test: Developers of Human Blood Substitutes Seek to Carve Out a Niche for First-Generation Products, Chem. & Engg. News, 76 (20): 37-44, 1998. (b) Winslow, R. M., Vandegriff, K. D., and Intaglietta, M., Blood Substitutes: New Challenges, Birkhäuser, Boston, 1996. (c) Winslow, R. M., Vandegriff, K. D., and Intaglietta, M., Blood Substitutes: Physiological Basis of Efficacy, Birkhäuser, Boston, 1995. (d) see Abstracts of the VI International Symposia on Blood Substitutes, Montreal, Quebec, Canada, August 5-7, 1996. (e) Kluger, R., Syntex Award Lecture, Anionic Electrophiles, Protein Modification, and Artificial Blood, Can. J. Chem., 72:2193, 1994. (f) Chang, T. M. S. and Geyer, R. P., Ed., Blood Substitutes, Marcel Dekker, Inc., New York, 1989.
2). Miller, I.F., CRC Crit. Rev. Bioengg., 149-177, 1978.
3). Bolin, R.B., Beyer, R.P., and Nemo, G.J. in Advances in Blood Substitutes Research, Alan R. Liss, Inc., New York, 1983.
4). Winslow, R. M., Hemoglobin-based Red Cell Substitutes, The Johns Hopkins University Press, Baltimore, 1992.
5). Boyiri, T., Safo, M. K., Danso-Danquah, R. E., Kister, J., Poyart, C., and Abraham, D. J., Biochemistry, 34: 15021, 1995. (b) DeVenuto, F. and Zegna, A., J. Surg. Res., 34:205, 1983.
6). Mok, W., Chen, D. -E., and Mazur, A., Fed. Proc., 34:1458, 1975.
7). (a) Snyder, S.R., Welty, E.V., Walder, R.Y., Williams, L.A., and Walder, J.A., Proc. Natl. Acad. Sci. USA, 84:7280, 1987. (b) Zhang, Q. and Olsen, K. W., Biochem. Biophys. Res. Commun., 203:1463, 1994. (c) Huang, H. and Olsen, K. W., Artif. Cells. Blood Subst. Immob. Biotech., 22:719, 1994. (d) Yang, T. and Olsen, K. W., Biochem. Biophys. Res. Commun., 174:518, 1991. (e) Yang, T. and Olsen,K. W., Biochem. Biophys. Res. Commun., 163:733, 1989. (f) Yang, T. and Olsen, K. W., Hemoglobin, 13:147, 1989.
8). (a) Kluger, R., Song, Y., Wodzinska, J., Head, C., Fujita, T., and Jones, R.T., J. Am. Chem. Soc., 114:9275, 1992. (b) Jones, R.T., Shih, D.T., Fujita, T.S., Song, Y., Xiao, H., Head, C., and Kluger, R., J. Biol. Chem., 271:675, 1996. (c) Zheng, Y. and Olsen, K. W., Artif. Cells. Blood Subst. Immob. Biotech., 24:587, 1996. (d) Paal, K., Jones, R. T., and Kluger, R. J. Am. Chem. Soc., 118:10380, 1996.
9). Kavanaugh, M.P., Shih, D. T. -B., and Jones, R.T., Biochemistry, 27:1804, 1988.
10). Hosmane, R. S. and Bertha, C. M., Biochem. Biophys. Res. Commun., 166: 567, 1990.
11). (a) Benesch, R.E. and Kwong, S., Biochem. Biophys. Res. Commun., 156:9, 1988. (b) Benesch, R.E. and Kwong, S., J. of Protein Chem., 10:503, 1991. (c) Benesch, R.E. and Kwong, S., J. Biol. Chem., 265:14881, 1990.
12). (a) Kluger, R., Wodzinska, J., Jones, R. T., Head, C., Fujita, T., and Shih, D. T., Biochemistry, 31:7551, 1992. (b) Jones, R. T., Head, C. G., Fujita, T. S., Shih, D. T., Wodzinska, J., and Kluger, R., Biochemistry, 32:215, 1993. (c) Kluger, R., Shen, L., Xiao, H., and Jones, R. T., J. Am. Chem. Soc., 118:8782, 1996.
13). Crystal coordinates of carbonmonoxy HbA0 were employed for the modeling studies; see Silva, M. M., Rogers, P. H., and Arnone, A., J. Biol. Chem., 267:17248,1992.
14). Hosmane, R. S., Peri, S. P., Bhadti, V. S., and V. W. Macdonald, Bioorg. Med. Chem.., 6: 767, 1998.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0028 as the message subject of your e-mail.