[A0030]
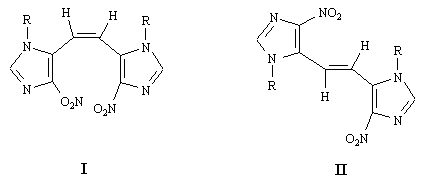
Novel Imidazole Analogues of Stilbene: Synthesis and Characterization of Cis- and Trans-1,2-bis(4-nitro-1-p-methoxybenzylimidazol-5-yl)ethene
Saika Siddiqui and Ramachandra, S. Hosmane*
Laboratory for Drug Design and Synthesis, Department of Chemistry
& Biochemistry
University of Maryland, Baltimore County (UMBC), 1000 Hilltop Circle
Baltimore, Maryland 21250, USA
E-mail: [email protected]
Received: 17 August 1999 / Uploaded: 21 August 1999
Keywords: Synthesis, Imidazole Analogues of Stilbene, Versatile Synthetic Intermediates.
INTRODUCTION
Stilbenes are an important class of organic compounds carrying a wide variety of useful
applications in organic synthesis. These applications include, but are not limited to,
asymmetric dihydroxylation, photocyclization, photodimerization, and synthesis of diphenyl
acetylenes, bromohydrins, and benzils. Heterocyclic analogues of stilbenes are of interest
not only for exploring these various applications but also for their inherent potential to
serve as key precursors to the synthesis of a host of novel as well as known heterocycles
containing fused imidazole ring systems. We present here the synthesis and
characterization of the title cis- and trans-1,2-bis(4-nitro-1-p-methoxybenzylimidazol-5-yl)ethene,
I and II.

RESULTS & DISCUSSION
The synthesis of title compounds I and II started with 4-methylimidazole, which was nitrated1 using a mixture of concentrated sulfuric and nitric acids to obtain 5-methyl-4-nitro-1H-imidazole (1) (Scheme 1). The crude nitrated product was treated with para-methoxybenzyl chloride and potassium carbonate in DMF to yield crystals of 4-nitro-1-p-methoxybenzyl-5-methyl-1H- imidazole (2) as a single regioisomer, as determined from NMR data. The regioisomeric assignment of 2 was based upon (a) comparison of the benzyl absorptions of 2 in its 1H NMR spectrum with those of several other benzyl-substituted nitroimidazoles previously synthesized in this laboratory,2 and (b) the established fact that the alkylation of nitroimidazoles predominantly yield substitutions at the nitrogen atom farther away from the nitro group.3
SCHEME 1
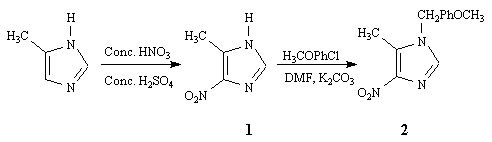
The target dimers I and II were prepared (Scheme 2) by the reaction of 2 with N-chlorosuccinimide (NCS)4 in the presence of potassium tert-butoxide in DMF. The same result was obtained with N-bromosuccinimide (NBS). The ratio of cis (I) and trans (II) isomers in the
SCHEME 2

product mixture depended upon the mode of addition of the reactants. Thus, when a solution of NCS in DMF was added to the mixture of 2 and potassium tert-butoxide in DMF, both I and II formed, and the yield was poor, perhaps due to the formation of polymers in addition to the dimers. On the other hand, the trans isomer II was the major product upon slow, reverse addition of 2 and potassium tert-butoxide to a solution of NCS in DMF. The 1H NMR of the product mixture obtained by direct addition of NCS (the first method described above) exhibited two sets of peaks, while that obtained by the reverse addition showed only a single set of peaks.
Figure 1: The UV absorbance spectrum of the mixture of I and II in chloroform: max: 1: 370; 2: 310; 3: 242; 4: 210 nm
The structural distinction between the two geometric isomers was achieved by comparing
the UV absorbance data of the mixture I and II with that
of II alone. The UV absorbance spectra of I and II,
depicted in Figure 1 showed two peaks above 300 nm, one at max 310
nm and 370 nm. The UV absorbance spectra of II in Figure 2,
on the other hand, showed only one peak at max 370 nm. The cis
isomer I, with its two-charged nitro groups in close proximity, is
expected to absorb at a lower wave number (higher energy) than the trans isomer II.
Thus I and II were assigned the cis and trans
configurations, respectively. The mass spectrometric and microanalytical data were also
consistent with the dimeric structures of I and II.
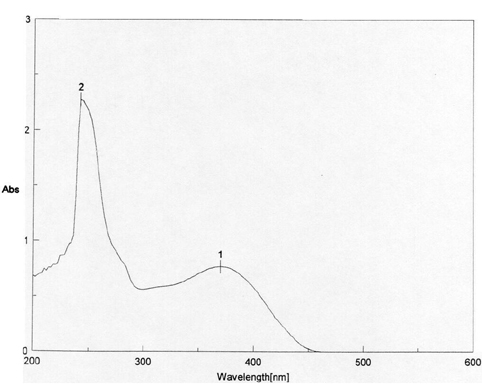
Figure 2: UV absorbance spectrum of II in chloroform. max: 1: 370; 2: 242 nm.
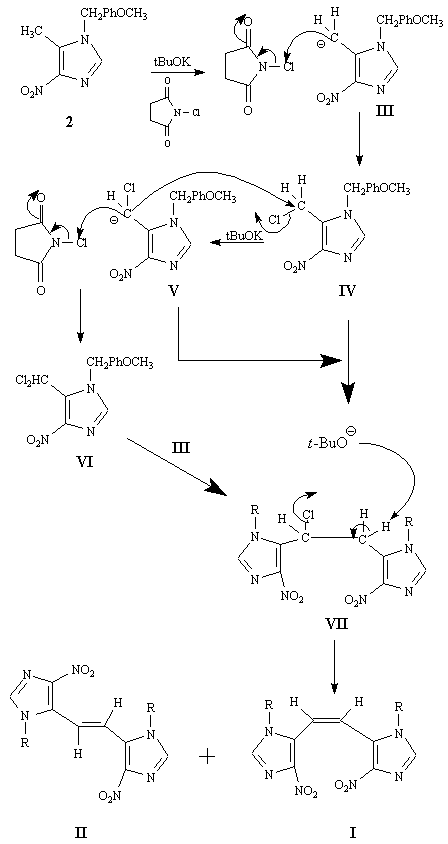
A tentative reaction pathway for the formation of I and II from 2 is outlined in Scheme 3. It appears that the key intermediate is the monochloro dimer VII, which might be formed via a couple different routes, including the nucleophilic displacement reaction of the carbanion V on the initially formed monochloro species IV or by the attack of carbanion III on the dichloro species VI. The intermediate VII undergoes further dehydrohalogenation in the presence of potassium t-butoxide to yield the observed cis and trans dimers I and II, respectively.
Schmeme 3
ACKNOWLEDGMENT
This work was supported by a grant (# RO1 CA71079) from the National Institutes of Health.
REFERENCES
1). Allsebrook, W. E; Gulland, J. M.; Story L. F., "The Constitution of Purine Nucleosides. Part X. A New Synthesis of Xanthineand Attempted Synthesis of Xanthine Glucosides and Glyoxalines," J. Chem. Soc. 1942, 232-236.
2). Hosmane, R. S.; Bhan, A., "The Synthesis of Ring-expanded Analogues of Xanthine, Containing the Imidazo[4,5-e][1,4]diazepine Ring System," J. Heterocycl. Chem. 1990, 27, 2189-2196.
3). Vaidya, V. P.; Hosmane, R. S.; Siriwardane, U.; Zhang, H.; Hosmane, N. S., "Unequivocal Structural Assignment of the Product of Methylation of 4-Nitro-5-styrylimidazole," Struct. Chem.1993, 4, 339-343.
4). Vaz, A. D. N.; Schoellmann G., "A Convenient and Simple Method for '-Chlorination of , and Conjugated Ketones," J. Org. Chem. 1984, 49, 1286-1288.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0030 as the message subject of your e-mail.