[A0032]
A Versatile Synthetic Precursor for Introduction of
Specific N6-Modifications in 2,6-Diaminopurine
Nucleosides:
N2-Acetyl-2',3',5'-tri-O-acetyl-N6-(1,2,4-triazol-1-yl)-
2,6-diaminopurine-9--D-ribofuranoside
XiaoHua Xu, Zhiyuan Sun, 2 and Ramachandra, S. Hosmane 1, *
1 Laboratory for Drug
Design and Synthesis, Department of Chemistry & Biochemistry
University of Maryland, Baltimore County (UMBC),
1000 Hilltop Circle
Baltimore, Maryland 21250, USA
E-mail: [email protected]
and
2Research Institute of Elemento Organic
Chemistry, Nankai University
94 Weijin Road, Tianjin 300071, Peoples Republic of
China
Received: 20 August 1999 / Uploaded: 23 August 1999
Keywords: 2,6-Diaminopurine Nucleosides, Versatile Synthetic Precursor for Specific N-6 Modifications, Dimers of 2,6-Diaminopurine Nucleosides with Methylene Bridges, Antiviral and Antitumor Compounds
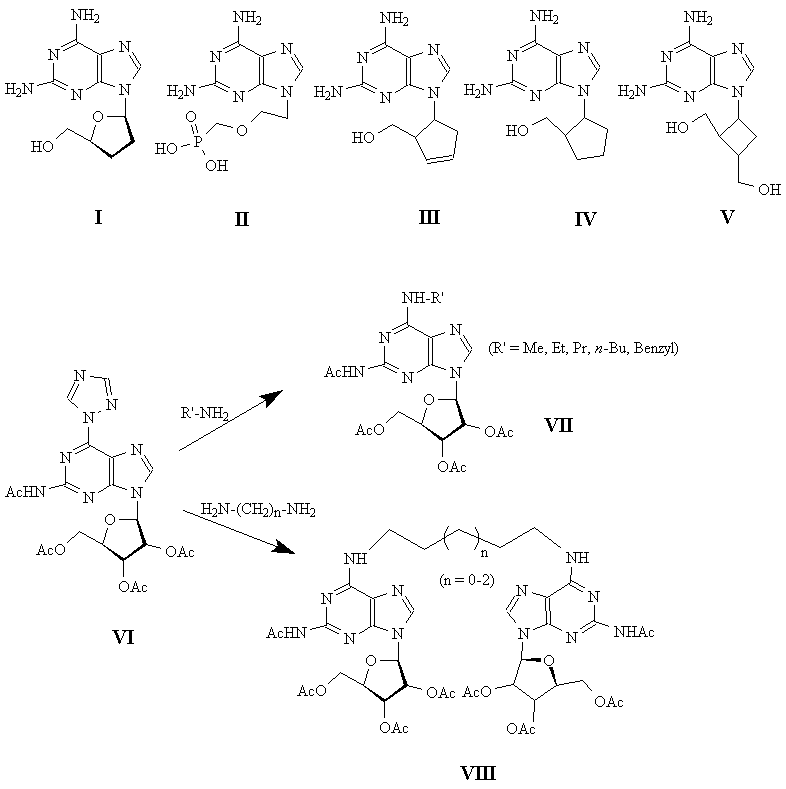
2,6-Diaminopurines as well as their nucleoside and nucleotide analogues have attracted considerable attention in recent years as potential antiviral and antitumor compounds. Some of these compounds have already been shown to be potent anti-HIV agents, including 2',3'-dideoxy-2,6-diaminopurine-9--D-ribofuranoside (I), the acyclic nucleotide analogue 9-[2-(phosphonylmethoxyethyl)]-2,6-diaminopurine (II), and the three carbocyclic nucleoside analogues (±)-cis-[4'-(2,6-diamino-9H- purin-9-yl)-2-cyclopentenyl]carbinol (III), (±)-cis-[3'-(2,6-diamino-9H-purin-9-yl)cyclopentyl] carbinol (IV), and (±)-9-[2',3'-bis(hydroxymethyl)]cyclobutyl-2,6-diaminopurine (V). As part of a program to improve profiles of drug efficiency and toxicity of 2,6-diaminopurine nucleoside analogues, it became necessary to explore the structure-activity relationships (SAR) via specific modifications at the N6-position of 2,6-diaminopurine ring. However, all available conventional methods to accomplish this goal, including the alkylation of the N6-amino group of 2,6-diaminopurine riboside or the displacement of a halogen group of 2-amino-6-chloropurine riboside yielded intractable mixtures of products and/or poor yields. We report here the synthesis of a versatile, highly reactive precursor (VI), which upon reaction with amine nucleophiles, gave the desired, specifically N6-modified 2,6-diaminopurinepurine nucleosides (VII) in high yields. In addition, the reaction of VI with polymethylenediamines afforded the polymethylene-bridged dimers of 2,6-diaminopurine nucleosides (VIII).

All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0032 as the message subject of your e-mail.