[A0033]
Base-Pairing Studies of a Ring-Expanded ("Fat") Nucleoside Analogue Possessing Potent Antiviral Activity
Maria Bretner, Huanming Chen, and Ramachandra, S. Hosmane*
Laboratory for Drug Design and Synthesis, Department of Chemistry
& Biochemistry
University of Maryland, Baltimore County (UMBC), 1000 Hilltop Circle
Baltimore, Maryland 21250, USA
E-mail: [email protected]
Received: 20 August 1999 / Uploaded: 23 August 1999
Keywords: 1H NMR Studies, Base-Pairing Properties, Ring-Expanded ("Fat" Nucleoside
Ring-expanded ("fat") nucleosides and nucleotides are potentially useful probes for nucleic acid metabolism, structure, and function. With their structural resemblance to natural purines, they are a rich source of substrates or inhibitors of enzymes of nucleic acid metabolism as well as of those requiring energy cofactors such as ATP or GTP. As ring-expansion is anticipated to considerably affect the electronic, spatial, and geometric characteristics, they are also excellent probes for steric and conformational constraints of nucleic acid double-helices.
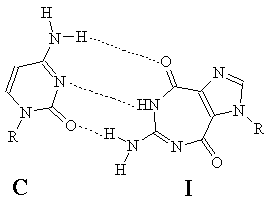
The ring-expanded nucleoside I that we recently synthesized showed potent anti-hepatitis B virus activity in vitro in submicromolar range with practically no toxicity even up to tens of thousands of times the therapeutic concentration levels. As part of a program to explore the mechanism of biological activity of I, it became necessary to investigate the base-pairing properties of I with appropriate pyrimidine partners. Since I can exist in several tautomeric forms in solution, it is theoretically capable of base-pairing with either cytidine (C) or Uridine (U). We present here the results of our extensive 1H NMR studies, which suggest that I base-pairs strongly with C, but not with U. These results further imply that the predominant tautomeric form of I is amino-diketo form as depicted below.

All comments on this poster should be sent by e-mail to (mailto:[email protected])
[email protected] with A0033 as the message subject of your e-mail.