[A0035]
A New, Improved Synthesis of 9-Benzyladenine: An Important Heterocyclic Analogue of Adenosine Useful for Chemical and Biochemical Research of Nucleic Acids
Zhiyuan Sun, ¶, § and Ramachandra, S. Hosmane §, *
§ Laboratory for Drug Design and Synthesis, Department of
Chemistry & Biochemistry
University of Maryland, Baltimore County (UMBC), 1000 Hilltop Circle
Baltimore, Maryland 21250, USA
E-mail: [email protected]
and
¶ Research Institute of Elemento Organic Chemistry, Nankai University
94 Weijin Road, Tianjin 300071, Peoples Republic of China
Received: 20 August 1999 / Uploaded: 25 August 1999
Keywords: Improved Syntheses of 9-Benzyladenine and 5-Amino-4-cyano-1-benzylimidazole
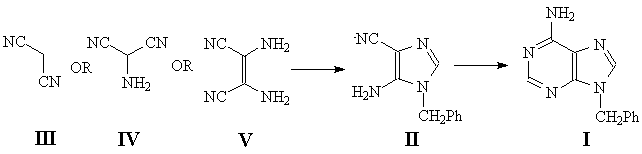
9-Benzyladenine (I) is a rare chemical often used by chemists, biochemists, as well as biophysical chemists doing research in the field of nucleic acids as a convenient heterocyclic base model for physicochemical comparison with adenosine. However, I is commercially unavailable, and the synthetic methods available from the literature are tedious and poor yielding. We have synthesized I from 5-amino-1-benzyl-4-cyanoimidazole (II) which, in turn, was synthesized from acyclic precursors, III, IV or V, using three different methods. We report herein all these methods, along with our recommendation for the best method in terms of both convenience and product yield to access both the imidazole precursor II and the target 9-benzyladenine (I).

All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0035 as the message subject of your e-mail.