[A0040]
Abstract: Six-membered cyclic thioureas can be prepared by a convenient stereoselective method based on the reduction of readily available 4-hydroxy(or 4-alkoxy)hexahydropyrimidine-2-thiones or 1,2,3,4-tetrahydropyrimidine-2-thiones with NaBH4 - CF3COOH. Alternative method of the preparation of the target compounds includes reaction of 4-azido-, 4-acetoxy- or 4-arylsulfonylhexahydropyrimidine-2-thiones with NaBH4.
Keywords: hexahydropyrimidine-2-thiones/ones,
1,2,3,4-tetrahydropyrimidine-2-thiones, tetrahydro-1,3-thiazine-2-thiones, sodium
tetrahydroborate - trifluoacetic acid
![]() Introduction
Introduction
![]() Results
and Discussion
Results
and Discussion
![]() Conclusion
Conclusion
![]() References
References
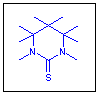
Six-membered cyclic thioureas, namely hexahydropyrimidine-2-thiones 1, are currently of interest due to their biological activity and other useful properties [1]. The application of the substances in organic synthesis has been described [2]. Besides, these compounds are valuable objects for spectroscopic and theoretical investigations.
The general method for synthesis of hexahydropyrimidine-2-thiones includes condensation
of (N-C-C-C-N + C)- type. For example, they are prepared by cyclization of
1,3-diamines with thiophosgene, carbon disulfide, etc. [1]. However, this method suffers
from the fact that rather often the starting 1,3-alkanediamines can not be easily
obtained, especially in a stereoselective manner. In contrast, various
4-hydroxyhexahydropyrimidine-2-thiones 2, 4-alkoxyhexahydropyrimidine-2-thiones 3
and 1,2,3,4-tetrahydropyrimidine-2-thiones 4 are readily available [3]. It is known
that these compounds react with nucleophiles to give the corresponding 4-substituted
hexahydropyrimidine-2-thiones [4]. We proposed that the reaction of 2-4 and some
other 4-substituted hexahydropyrimidine-2-thiones with H-nucleophiles could provide a
simple general method for the synthesis of the target compounds. We report here on new
convenient procedures for the preparation of six-membered cyclic thioureas by reduction of
4-hydroxy(or 4-alkoxy)hexahydropyrimidine-2-thiones,
1,2,3,4-tetrahydropyrimidine-2-thiones or some 4-functionally substituted
hexahydropyrimidine-2-thiones. As reducing reagents we used sodium tetrahydroborate or
sodium tetrahydroborate in the presence of carboxylic acids. Earlier the latter reducing
system was successfully applied for the transformation of a-hydroxyalkylamides
into N-alkylamides [5].
We found that the 4-hydroxyhexahydropyrimidine-2-thiones 2a-f are readily reduced by NaBH4 in the presence of CF3COOH [molar ratio of 2 : NaBH4 : CF3COOH 1 : (3-4) : (20-30)] (THF or 1,4-dioxane, r.t., 3 h) to form the corresponding hexahydropyrimidine-2-thiones 1a-f in 83-99 % isolated yields (Scheme 1).
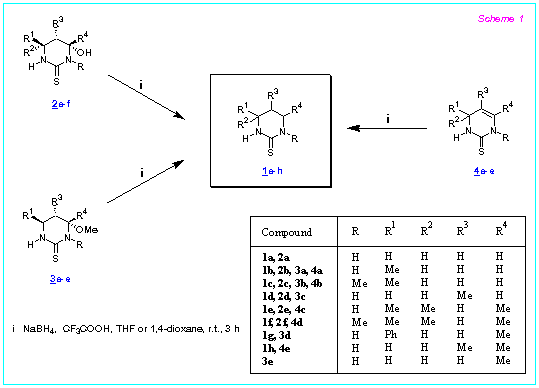
The reaction is usually carried out by the addition of CF3COOH to the suspension of the pyrimidine and NaBH4 in the solvent at 0 oC followed by stirring the reaction mixtures for 3 h at r.t. (method A). The reactions also proceed successfully with another order of the addition of the reagents: CF3COOH and then dry pyrimidine are added to the suspension of NaBH4 in the solvent cooled to 0 oC (method B) or NaBH4 is added to the mixture of the pyrimidine, CF3COOH and the solvent at 0 oC (method C).
4-Alkoxyhexahydropyrimidine-2-thiones 3 and 1,2,3,4-tetrahydropyrimidine-2-thiones 4 can equally well serve as the starting material for the synthesis of hexahydropyrimidine-2-thiones. Thus the reaction of alkoxypyrimidines 3a-e with NaBH4 - CF3COOH in the above mentioned conditions gives the compounds 1b-d,g in 80-99.5 % yields. Similarly, the reduction of the tetrahydropyrimidines 4a-e affords the compounds 1b,c,e,f,h in 82-98 % yields. It should be mentioned that the reduction of both 3a and 3e results in the formation of the same pyrimidine 1b.
The reduction of compounds 2-4 proceeds in a diastereoselective manner. For example, the reduction of the methoxypyrimidine 3d possessing two chiral carbon atoms results in the formation of the compound 1g which is a mixture of the cis and trans diastereoisomers (96:4 respectively). Analogously, achiral tetrahydropyrimidine 4e is converted into the compound 1h as a mixture of cis and trans isomers (24:76) where trans isomer is predominant.
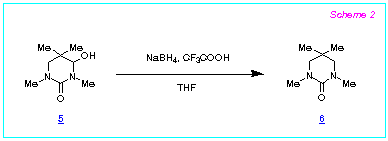
The reduction with NaBH4 - CF3COOH is
highly efficient as well for the synthesis of six-membered cyclic ureas, namely
hexahydropyrimidine-2-ones, starting from the corresponding 4-hydroxy derivatives, as we
showed by the conversion of the hydroxypyrimidinone 5 to the compound 6 in
95 % isolated yield (Scheme 2).
Evidently, NaBH4 - CF3COOH can be also applied
for the reduction of other nitrogen containing heterocycles possessing amidoalkylation
properties. For example, cyclic dithiocarbamates, namely the
tetrahydro-1,3-thiazine-2-thiones 8a,b, are obtained in 79 and 93 % yields
respectively by the reduction of the 4-methoxytetrahydro-1,3-thiazine-2-thiones 7a,b
with NaBH4 - CF3COOH according to the method
A (Scheme 3).
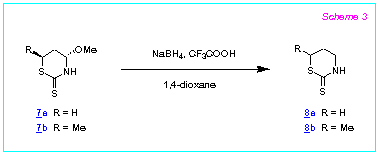
We found that the reductive ability of the NaBH4 - RCOOH system
decreases sharply when CF3COOH is displaced by the weaker acetic
acid. Actually, the reduction of the compounds 2b, 4d with NaBH4 - CH3COOH in THF does not practically occur.
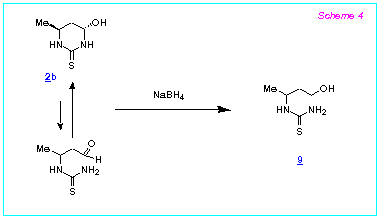
The reaction of 4-hydroxyhexahydropyrimidine-2-thiones with NaBH4
proceeds differently without the addition of CF3COOH. Thus, the
reaction of the compounds 2b with NaBH4 (water, 50 oC) gives the product of the reduction of the aldehyde group of
the acyclic isomeric form of 2b, namely the N-(4-hydroxybut-2-yl)thiourea 9
(93% yield) (Scheme 4).
Thus, the first stage of the reactions studied is probably the generation of the
acylimmonium cations from 2-4 in the presence of CF3COOH.
These cations are further subjected to nucleophilic attack at the C(4) position by the
reducing agent, sodium tris(trifluoroacetoxy)hydroborate, formed by the reaction of NaBH4 with CF3COOH.
We proposed that it could be possible also to prepare six-membered cyclic thioureas by reduction of 4-substituted hexahydropyrimidine-2-thiones, bearing more easily leaving group at the C(4) position than hydroxy or alkoxy group, with NaBH4 even in absence of CF3COOH. Our preliminary investigations showed that 4-azido-, 4-acetoxy and 4-arylsulfonylhexahydropyrimidine-2-thiones can readily react with various nucleophiles under mild reaction conditions to produce the corresponding 4-substituted products [6]. Thus we studied reactions of the above mentioned compounds with NaBH4.
We found that the 4-azidohexahydropyrimidine-2-thiones 10a-d readily react with NaBH4 in acetonitrile or DMFA at r.t. to afford the compounds 1a-c,i in 89-100 % isolated yields. Reduction of 4-acetoxyhexahydropyrimidine-2-thiones 11a,b and 4-phenylsulfonylhexahydropyrimidine-2-thione 12 with NaBH4 in acetonitrile proceeds also easily and gives the compounds 1b,c (Scheme 5).
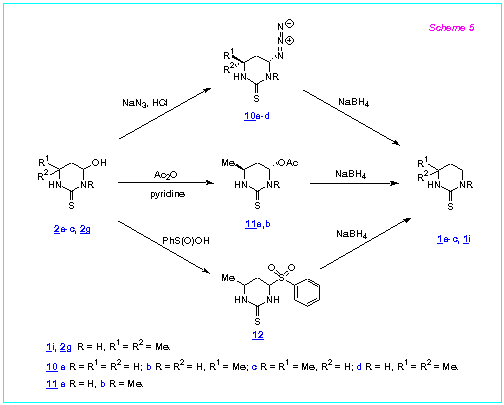
The starting azidopyrimidines 10a-d and phenylsulfonylpyrimidine 12
are obtained by reaction of the corresponding hydroxypyrimidines 2a-c,g with
hydrazoic acid or bensenesulfinic acid in water (r.t., 24 h) in yields more than 90 %. The
acetoxypyrimidines 11a,b are produced in 81-85 % yields by treatment of 2b,c
with Ac2O in pyridine (r.t., 12 h).
Thus the present work shows that six-membered cyclic thioureas can be easily prepared
according to two general procedures. The first route is stereoselective and includes
direct reduction of readily available 4-hydroxy(or 4-alkoxy)hexahydropyrimidine-2-thiones
and 1,2,3,4-tetrahydropyrimidine-2-thiones with NaBH4 - CF3COOH. The second procedure lies in synthesis of 4-azido-, 4-acetoxy- or
4-arylsulfonylhexahydropyrimidine-2-thiones followed by their reduction with NaBH4. Both the methods are very flexible. They give possibility to prepare
not only a large number of cyclic thioureas but also related compounds, such as cyclic
ureas, cyclic dithiocarbamates, etc.
1. Bogatskii, A.V.; Luk'yanenko, N.G.; Kirichenko, T.I. Khim. Geterotsicl. Soedin. (Rus), 1983, 723 and references cited therein.
2. Sharma, S.D.; Arora, S.K.; Mehra, U. Indian J. Chem., 1985, 24, 895; Chadha, V.K. J. Indian Chem. Soc., 1977, 54, 878; Arya, V.P.; Shenoy, S.J. Indian J. Chem., 1976, 14, 759; Chaudhary, H.S.; Pujari, H.K. ibid, 1972, 10, 766.
3. Ignatova, L.A.; Shutalev, A.D.; Shingareeva, A.G.; Dymova, S.F.; Unkovskii, B.V. Khim. Geterotsikl. Soedin. (Rus), 1985, 260; Shutalev, A.D.; Ignatova, L.A.; Unkovskii, B.V. ibid, 1984, 548; Zigeuner, G.; Galatik, W.; Lintschinger, W.-B.; Wede, F. Monatsh. Chem., 1975, 106, 1219; Ovechkin, P.L.; Ignatova, L.A.; Unkovskii, B.V. Khim. Geterotsikl. Soedin., 1972, 941; Zigeuner, G.; Frank, A.; Dujmovits, H.; Adam, W. Monatsh. Chem., 1970, 101, 1415; Unkovskii, B.V.; Ignatova, L.A.; Zaitseva, M.G. Khim. Geterotsikl. Soedin. (Rus), 1969, 889; Zimmermann, R.; Brahler, B.; Hotze, H. Pat. 1065849 BRD (1961).
4. Shutalev, A.D.; Alekseeva, S.G. Khim. Geterotsikl. Soedin. (Rus), 1995, 377; Shutalev, A.D.; Pagaev, M.T.; Ignatova, L.A. ibid, 1994, 1093; Shutalev, A.D.; Komarova, E.N.; Pagaev, M.T.; Ignatova, L.A. ibid, 1993, 1259; Shutalev, A.D.; Ignatova, L.A. ibid, 1991, 228; Ignatova, L.A.; Shutalev, A.D.; Pagaev, M.T.; Unkovskii, B.V. ibid, 1988, 234.
5. Gribble, G.W.; Nutaitis, C.F. Org. Prep. Proced. Intern. 1985, 17, 317.
6. Shutalev, A.D. Khim. Geterotsikl. Soedin. (Rus), 1993, 1389; Shutalev, A.D.; Komarova, E.N.; Pagaev, M.T.; Ignatova, L.A. ibid, 1993, 1259; Shutalev, A.D.; Ignatova, L.A. ibid, 1991, 228; Shutalev, A.D.; Ignatova, L.A.; Unkovskii, B.V. ibid, 1990, 133.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0040 as the message subject of your e-mail.