[B0002]
Polyhedral Oligosilsesquioxanes as
Biological Scaffolds
Kevin D. Wyndhama, Frank J. Feherb
Department of Chemistry, University of California,
Irvine, California 92697-2025
a [email protected]; b [email protected]; http://chem.ps.uci.edu/~fjfeher/FJFgroupHome.html
Received: 9 August 1999 / Uploaded: 10 August 1999
![]()
Abstract |
Development of polyhedral oligosilsesquioxanes as scaffolds for biologically relevant groups (e.g., peptides and carbohydrates). Selective mono- and difunctionalization of these frameworks with biologically relevant groups offers potential drug delivery vehicles. |
![]()
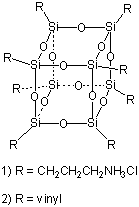 Symmetric core scaffolds possessing multiple reactive functional
groups have recently attracted much interest as templates for the presentation of
molecular domains of biological relevance. In principle, any polyfunctional molecule may
serve as a scaffold to present multiple copies of a biologically relevant pendant group,
but the most attractive cores are those that have particular geometrical parameters (e.g.,
size, shape, symmetry) to allow unique ligand presentation. Here, we describe the first
use of polyhedral oligosilsesquioxanes as scaffold for peptides, carbohydrates
and the development of these frameworks as site-specific drug delivery vehicles.
Symmetric core scaffolds possessing multiple reactive functional
groups have recently attracted much interest as templates for the presentation of
molecular domains of biological relevance. In principle, any polyfunctional molecule may
serve as a scaffold to present multiple copies of a biologically relevant pendant group,
but the most attractive cores are those that have particular geometrical parameters (e.g.,
size, shape, symmetry) to allow unique ligand presentation. Here, we describe the first
use of polyhedral oligosilsesquioxanes as scaffold for peptides, carbohydrates
and the development of these frameworks as site-specific drug delivery vehicles.
Polyhedral oligosilsesquioxanes are a unique class of Si/O clusters synthesized from the hydrolytic condensation of trifunctional organ silicon monomers. The octaamine framework (i.e., 1) is readily available as the hydrochloride salt from the acid catylized condensation of g-aminopropyl triethoxysilane. Reactions of 1 with a variety of electrophiles, including anhydrides, lactones, acid chlorides and isocyanates afforded new families of functionalized R8Si8O12 frameworks in excellent yields.1,2 The octavinyl cage (i.e., 2) can be obtained by condensation of CH2=CHSiCl3. This core has been functionalized by radical addition of phospines and thiols, epoxidation and cross-metathesis with a wide variety of alkenes.3
Peptidyl
Functionalized Silsesquioxanes
Octaamine 1 undergoes octafunctionalization with N-protected amino acids and N-protected di- and tri-peptides under standard coupling conditions.4 All reactions can be easily monitored by NMR (1H, 13C , 29Si) spectroscopy as well mass spectrometry (MALDI-TOF), and protected products can be easily purified by precipitation in aqueous acid. Deprotection of the Cbz-protecting group can be easily achieved by catalytic hydrogenolysis allowing deprotected products to be otained in excellent yields. These frameworks can then be used in iterative peptide synthesis to generated di- and tri-peptide functionalized frameworks.
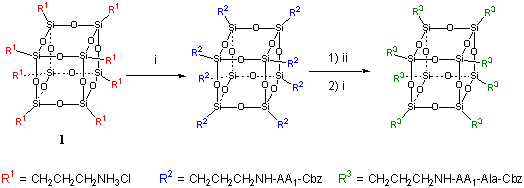
AA1 = Pro, Ala, Leu-Phe, Ala-Leu-Phe
Scheme 1. Reagents and
conditions: i protected amino acid (1.2-4 equivalents per amine), TBTU (2-4
equiv. per amine), HOBt (4 equiv. per amine) and (i-Pr)2NEt (9 equiv. per
amine) in DMF, 10-24 h, 25 °C; ii, Hydrogen (100 psi), 10% Pd/C, 1 m HCl/MeOH, 8
h, 25 °C.
Carbohydrate Functionalized
Silsesquioxanes
Carbohydrate functionalized frameworks can be synthesized by
reactions of 1 with carbohydrate derived lactones (e.g., lactonolactone or
gluconolactone) afford new frameworks containing eight equivalent galactose-sustituents
(i.e., 3) or eight equivalent glucosyl terminated framework (i.e., 4).5
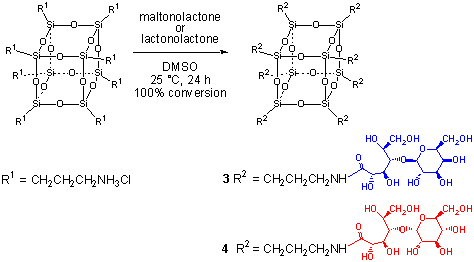
Scheme 2. Reagents and conditions:
Framework 1 is initally neutralized by Amberlite IRA-400 (OH-) ion exchange resin
in DMSO before addition of lactonolactone or gluconolactone. The product is purified by
dyalisis (1000 d cutoff) against water (4 x 4 L).
These frameworks demonstrate biological activity having specific binding with carbohydrate specific binding proteins called lectins. The asialoglycoprotein receptor (ASGPR) is an integral mammalian hepatocyte mambrane lectin which has selctive binding to terminal non-reducing b-D-galactopyranosyl residues and demonstrates increased binding with a specific geometric arrangement of antennary b-D-galactopyranosyl groups. Enhanced binding was observed with octagalactosyl framework 3 in an inhibition experiment of the ASGPR with a radioactively labeled neoglycoprotein (135I-Asialoorosomucoid), while no inhibition was observed with frameworks 1 or 3 (See Figure 1).

Figure 1. Competitive inhibition of
HepG2-ASGPR mediated uptake of 125I-ASOM (1 µM, 0.5 mL/well) by 1 (?), 3 (?), and 4 (?) as measured by scintillation counting of the 125I
label. The reaction was performed in binding media (0.5 mL/well, pH = 7.4), incubated at
37 °C for 2 h before lysis with 10% SDS (0.5 mL/well). The inhibitory potency (IC50)
for unlabelled ASOM was determined to be 0.65 µM from a separate control experiment.
Monofunctionalization of Silsesquioxanes
The ability to differentiate of one or more peripheral groups has important implications for design of site specific drug conjugates. Selective monofuncitonalization of a polyfunctional molecule is extremely difficult resulting in of complex mixtures of products. For example the reaction of trific acid (HOTf) with vinyl silsesquoxane results in a mixture partially of b-triflate functionalized frameworks.6
![]()
Individual products (e.g. 5a, 5b, 5c, 5d) could not be isolated from the crude reaction mixture by chromatography or recrystallization. However these b-triflates reacts with a variety of nucleophiles, including water or ammonia, to give rise to new mixtures of b-functionalized products (e.g., 6a-d, 7a-d) which can be separated by chromatography to afford individual products in multigram quantities.
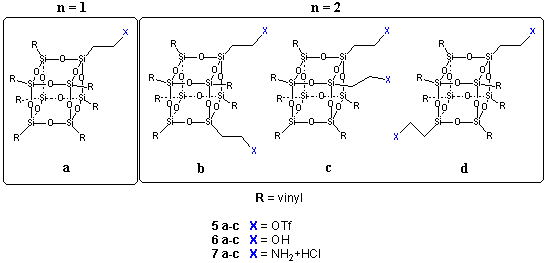
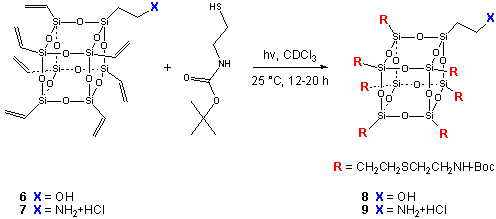
These frameworks can then be functionalized by transformation of the vinyl groups (e.g., by cross metathesis or thiol addition) to afford new cores to couple drugs, tags or targeting moieties. For example the UV catalyzed addition of thiols allows a facile means to add a protected amine to frameworks 6a or 7a to afford mono-alcohol or mono-amine frameworks 8 and 9.
Conclusions
We have synthesized a variety of polehedral silsesquioxanes to organize ensembles of biologically relevant motifs. Peptides can be attached to octaamine 1 in a convergent or divergent fashion. By reacting 1 with sugar-lactones, carbohydrate-functionalized silsesquixoxanes care formed which have specific binding with carbohydrate specific binding proteins. Finally the ability to synthesize R17R2Si8O12 frameworks have exciting implications for molecular recognition and design of new site-specific drugs.
* and
references contained within.