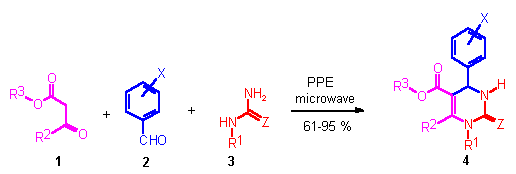
Scheme 1
[B0008]
A Novel High-Speed Method for the Generation of 4-Aryldihydropyrimidine Compound Libraries Using a Microwave-Assisted Biginelli Condensation Protocol.
C. Oliver Kappe*a and Rajender S. Varmab
a Institute of Organic Chemistry, Karl-Franzens-University Graz, A-8010
Graz, Austria.
Fax: ++43-316-3809840 http://www-ang.kfunigraz.ac.at/~kappeco
b U.S. Environmental Protection Agency National Risk Management Research
Laboratory
26 West Martin Luther King Drive, MS 443 Cincinnati, Ohio 45268, USA
Tel:(513)-487-2701, Fax:(513)-487-2514, E-mail: [email protected]
Received: 10 August 1999 / Uploaded: 11 August 1999
Abstract
In this presentation we report the application of microwave assisted chemistry to the parallel synthesis of 4-aryl-3,4-dihydropyrimidin-2(1H)-ones employing a solventless Biginelli multicomponent condensation protocol. The novel method employs neat mixtures of ▀-ketoesters, aryl aldehydes, and urea derivatives with polyphosphate ester (PPE) being used as reaction mediator. Irradiation of these mixtures for 90 s in an unmodified household microwave oven provides dihydropyrimidones in 61-95% yield after aqueous work-up. This protcol was extended towards the parallel synthesis of DHPM compound libraries.
Results
4-Aryl-3,4-dihydro-2(1H)-pyrimidone esters of type 4 ("Biginelli compounds", DHPMs) represent a heterocyclic system of remarkable pharmacological efficiency.[1] Appropriately functionalized DHPMs have emerged as potent calcium channel blockers, [2] antihypertensive agents, [3] selective a1a-adrenergic receptor antagonists,[4] and neuropeptide Y (NPY) antagonists.[5] The most straightforward protocol to synthesize DHPMs 4 involves the one-pot condensation of a ▀-keto ester 1, with an aryl aldehyde 2, and urea or thiourea derivative 3 under strongly acidic conditions (Biginelli condensation, Scheme 1).[1] In recent years, several combinatorial approaches towards DHPMs 4 have been advanced, using e.g. solid phase, or fluorous phase reaction conditions.[6]

Scheme 1
We have recently developed [7] a method that allows for the rapid and parellel synthesis of DHPMs 4 that does not rely on polymer-supported building blocks and therefore does not require the development of solid phase linking/cleaving chemistry. Instead, our procedure involves a microwave-promoted, solvent-free variation of the classical Biginelli condensation. The application of microwave (MW) irradiation in organic synthesis has been the focus of considerable attention in recent years and is becoming an increasingly popular technology.[8]
The microwave-expedited Biginelli reaction described herein is based on our recent finding that polyphosphate ester (PPE) serves as an excellent reaction mediator in the three-component Biginelli reaction.[9] In order to be able to carry out such Biginelli condensations in a faster and more efficient way we investigated the influence of MW irradiation on a neat mixture of ▀-keto ester 1, aryl aldehyde 2, (thio)urea derivative 3, and PPE. After some experimentation with respect to the molar ratios of reagents, and the irradiation time and power level of the MW set-up we have found a set of conditions that generally provides DHPMs in good to excellent yields (Table 1). These conditions employed a 1.1 : 1.0 : 3.0 molar ratio of ▀-keto ester 1, aryl aldehyde 2, and (thio)urea derivative 3, using 150 mg of PPE as reaction mediator. In a typical experiment the four reaction components are simply mixed in a glass beaker and irradiated in an unmodified household MW oven for a total of 90 s. During MW irradiation the reaction vessel is placed inside a larger container filled with alumina, which acts as a heat sink. After cooling, water was added to the reaction mixture which hydrolyzed PPE, dissolved excess (thio)urea and precipitated the solid DHPMs. For the majority of cases, the products obtained using this microwave/PPE-mediated Biginelli protocol had at least 95% purity (1H NMR, 200 MHz). For comparison purposes, literature yields obtained for DHPMs 4a-o using the traditional Biginelli reaction conditions (ethanol/catalytic HCl) are also given in Table 1. In the majority of cases a very significant increase (20-50%) in yield using the MW-induced protocol can be achieved. At the same time, the reaction time is reduced from typically 4-8 hours reflux (traditional heating) to a few minutes (MW irradiation).
The yields given in Table 1 refer to reaction runs at 2.0 mmol scale. For DHPM 4a a scale-up to 50 mmol (ca 13 g of product) was performed without any difficulties furnishing the desired DHPM in 88% yield after recrystallization. Apart from its simplicity and speed an important feature of the microwave-induced protocol is the ability to tolerate variations in all of the three building blocks. Thus, all five variable substituents around the DHPM scaffold 4 (R1-R3, X, Z) can be modified, increasing the structural diversity of DHPM analogs that can be synthesized expeditiously.
We have therefore extendend the scope of this microwave/PPE-mediated Biginelli procedure and have carried out the synthesis of a number of DHPM analogs in a parallel fashion in a single microwave irradiation experiment, following the MICROCOS, (Micowave-Assisted Combinatorial Synthesis) approach [11]. For this purpose e.g. 10 reaction vessels containing the appropriate mixtures of ▀-keto esters 1, aldehydes 2, urea 3, and PPE were placed inside an alumina bath (Figure 1a) and subsequently irradiated in the microwave oven (Figure 1b). After the usual aqueous workup the individual DHPMs were obtained in yields identical to the ones obtained in the conventional MW experiment (Table 1, [10]). This strategy is therefore clearly applicable to the parallel synthesis of single compound DHPM libraries. In view of the large number of commercially available or readily accessible aromatic aldehydes, ▀-keto esters, and urea derivatives large collections of DHPMs can potentially be prepared, applying the recently developed automated, high throughput robotic technologies for performing microwave-assisted combinatorial synthesis.[11]


Figure 1a Figure 1b
In conclusion, we have described a novel and highly efficient microwave-induced modification of the Biginelli multicomponent reaction that allows for the rapid assembly of structurally diverse DHPM derivatives. The advantages of this environmentally benign and safe protocol include a simple reaction set-up not requiring specialized equipment, high product yields, short reaction times, and the elimination of solvents or solid supports.
Acknowledgements: This work (C.O.K.) was supported by the Austrian Academy of
Sciences (APART 319) and the Austrian Science Fund (FWF, Project P-11994-CHE).
References and Notes
[1] For a review on DHPMs, see: Kappe, C. O. Tetrahedron1993, 49, 6937.
[2] Atwal, K. S.; Rovnyak, G. C.; Kimball, S. D.; Floyd, D. M.; Moreland, S.; Swanson, B. N.; Gougoutas, J. Z.; Schwartz, J.; Smillie, K. M.; Malley, M. F. J. Med. Chem. 1990, 33, 2629. Rovnyak, G. C.; Kimball, S. D.; Beyer, B.; Cucinotta, G.; DiMarco, J. D.; Gougoutas, J.; Hedberg, A.; Malley, M.; McCarthy, J. P.; Zhang, R.; Moreland, S. J. Med. Chem. 1995, 38, 119.
[3] Rovnyak, G. C.; Atwal, K. S.; Hedberg, A.; Kimball, S. D.; Moreland, S.; Gougoutas, J. Z.; O┤Reilly, B. C.; Schwartz, J.; Malley, M. F. J. Med. Chem. 1992, 35, 3254. Grover, G. J.; Dzwonczyk, S.; McMullen, D. M.; Normadinam, C. S.; Sleph, P. G.; Moreland, S. J. J. Cardiovasc. Pharmacol. 1995, 26, 289.
[4] Sidler, D. R.; Larsen, R. D.; Chartrain, M.; Ikemoto, N.; Roberge, C. M.; Taylor, C. S.; Li, W.; Bills, G. F. PCT Int. WO 99 07695.
[5] Bruce, M. A.; Pointdexter, G. S.; Johnson, G. PCT Int. Appl. WO 98 33,791.
[6] Wipf, P.; Cunningham, A. Tetrahedron Lett. 1995, 36, 7819. Robinett, L. D.; Yager, K. M.; Phelan, J. C. 211th National Meeting of the American Chemical Society, New Orleans, 1996; American Chemical Society: Washington, DC, 1996; ORGN 122. Studer, A.; Hadida, S.; Ferrito, R.; Kim, S.-Y.; Jeger, P.; Wipf, P.; Curran, D. P. Science 1997, 275, 823. Studer, A.; Jeger, P.; Wipf, P.; Curran, D. P. J. Org. Chem. 1997, 62, 2917. Lewandowski, K.; Murer, P.; Svec, F.; FrÚchet, J. M. J. Chem. Commun. 1998, 2237.
[7] Kappe, C. O.; Kumar, D.; Varma, R. S. Synthesis1999, in press.
[8] For recent reviews on microwave-assisted organic reactions, see: Varma, R. S. Green Chem. 1999, 43. Varma, R. S. Clean Products and Processes 1999, 1, 132. Loupy, A.; Petit, A.; Hamelin, J.; Texier-Boullet, F.; Jacquault, P.; MathÚ, D. Synthesis 1998, 1213 and ref cited therein.
[9] Kappe, C. O.; Falsone, F. S. Synlett 1998, 718.
[10] Parallel Synthesis of DHPMs 4a-o: The appropriate (see Table 1) ▀-keto esters 1 (1.1 mmol), aldehydes 2 (1.0 mmol), urea 3 (3.0 mmol) and PPE (150 mg) were placed in individual 10 mL glass beakers immersed in a crystallization dish (13.5 cm diameter) filled with alumina (400 g). This set-up was irradiated on the turntable in the MW oven 3 times at the 50% power level for 40 s with a 1 min and 2 min cooling period after the 1st and 2nd irradiation cycle, respectively. Aqueous work up as above provided DHPMs 4a-o in yields given in Table 1. A conventional (unmodified) houesehold microwave oven equipped with a turntable was used (Panasonic NN-3356/3306, 2450 MHz, 800 W.
[11] Cotterill, I. A.; Usyatinsky, A. Y.; Arnold, J. M.; Clark, D. S.; Dordick, J. S.; Michels, P. C.; Khmelnitsky, Y. L. Tetrahedron Lett. 1998, 39, 1117.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with b0008 as the message subject of your e-mail.