[B0009]
Coupling of Phenols to 2-Chlorotrityl Resins
under Non-basic Conditions
Bernd Renneberg1) and Werner Breitenstein2)
1)Argonaut Technologies AG, P.O. Box 43, CH-4132 Muttenz 2,
Switzerland
E-mail: [email protected]
2)Combinatorial Chemistry Unit, Core Technology Area, Novartis Pharma Inc., CH-4002 Basel, Switzerland
Received: 7 August 1999 / Uploaded: 18 August 1999
Introduction
In connection with the synthesis of combinatorial libraries starting from base labile phenolic compounds, which contain one or several aromatic hydroxy groups, we became interested in suitable methods for the coupling of phenols to solid supports under non-basic reaction conditions.
For regioselectivity reasons we mainly focused our investigations on the sterically demanding trityl linker. It is known that phenols can be attached to polymer bound trityl chloride and 2-chlorotrityl chloride using ethyl-diisopropylamine or pyridine as a base (1).
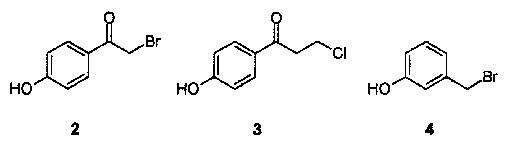
However, this method is not suitable for phenols containing functionalities, which are prone to side reactions under basic conditions, such as 4-hydroxyphenacyl bromide (2), 4-hydroxy-3�- chloropropiophenone (3) or 3-hydroxybenzyl bromide (4).
This prompted us to investigate a solid phase tritylation procedure, which can be carried out under non-basic reaction conditions.
Results and Discussion
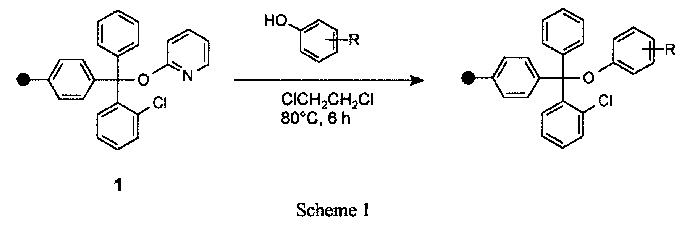
We found a simple method for the first synthesis of polymer-bound 2-pyridyl trityl ether (1) starting from commercially available 2-chlorotrityl chloride resin. The regioselective N- or O-tritylation of 2(1H)-pyridone and the reaction of the resulting products with alcohols and phenols in solution has been investigated by Effenberger et al. (2). Polymer 1 allows the convenient immobilisation of phenols under nearly neutral reaction conditions (Scheme 1). Only two or three equivalents of the phenolic compound are necessary for successful coupling.
Best loading levels are obtained in chlorinated solvents. The immobilisation is usually complete after 6-8 h at 80 �C. Addition of acid is not necessary, although a catalytic amount (5 mol%) of TFA increases the reaction rate considerably. A similar catalytic effect of acids is known from 2-pyridyl trityl ethers (2) in solution.

This coupling method using 1 as tritylation agent allows immobilisation of the base labile phenols 2-4 (Table 1) with reasonable recovery yields and purities after cleavage. The same reaction starting from 2-chlorotrityl chloride resin using Et3N as base leads to complex mixtures containing only traces of the desired products.
Table 1. Coupling and cleavage of base labile phenols.
Phenol |
Yield of recoverya) |
Purityb) |
|
| 2 | 78 % |
> 95 % | |
| 3 | 76 % |
> 95 % | |
| 4 | 77 % |
> 95 % c) | |
a) Crude yields after cleaving the phenols from the resin with TFA / triisopropylsilane / CH2Cl2 (5:5:90). Yields are based on the loading of resin 1.
To study scope and limitation of this new phenol tritylation procedure on solid supports, the phenols 5-12 were reacted with 1 (Table 2). The observed recovery yields vary from excellent to poor depending on the substituent pattern of the phenols used. Phenolic compounds containing an ortho methyl group or no substituent in that position gave the best results. The low yield obtained with 9 can be explained with the reduced reactivity of this compound due to hydrogen bond formation.
Table 2. Coupling and cleavage of various phenols
a) See footnote a) of Table 1. The purity of the recovered phenols 5-12 was > 95 % according to HPLC.
Conclusion
A convenient method for the coupling of phenols to 2-chlorotrityl resin under non-basic conditions has been developed using the polymer bound pyridyl ether derivative 1 as tritylation agent. Detachment of the phenols can be effected under mild conditions using TFA/CH2Cl2 5:95 (and triisopropylsilane if necessary).
The procedure is particularly well suited for the immobilisation of base sensitive phenolic compounds.
References
(1) see e.g.: Bernhardt A.; Drewello M.; Schutkowski M. J. Pept. Res. 1997, 50, 143-152. Takahashi T.; Ebata S.; Doi T. Tetrahedron Lett. 1998, 39, 1369-1372. Shankar B.B.; Yang D.Y.; Girton S.; Ganguly A.K. Tetrahedron Lett. 1998, 39, 2447-2448. Zhu Z.; Mckittrick B. Tetrahedron Lett. 1998, 39, 7479-7482. Haap W.J.; Kaiser D.; Walk T.B.; Jung G. Tetrahedron 1998, 54, 3705-3724.
(2) Effenberger, F.; Brodt, W.; Zinczuk, J. Chem. Ber. 1983, 116, 3011-3026.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with B0009 as the message subject of your e-mail.