[B0010]
Multi-step Split Synthesis on the TridentTM: Parallel Solution-Phase Synthesis of Urea and Amide Library
Sukanta Bhattacharyya, Lisa Tamanaha,
Lanchi Vo, Peter Wright and Jeff Labadie
Argonaut Technologies, 887 Industrial Road Suite G, San Carlos, CA 94070
E-mail: [email protected]
Received: 20 August 1999 / Uploaded: 29 August 1999
The Trident automated synthesizer was successfully employed in a two-step split
synthesis of amides, sulfonamides and ureas. The key synthetic step involved the
preparation of 24 secondary amines from 4 primary amines and 6 carbonyl compounds using
Ti(OiPr)4 and NaBH4. These amines were then used in 192 reactions to synthesize 96 ureas
in duplicate and 96 reactions to synthesize 48 amides and 48 sulfonamides. The reaction
sequences clearly demonstrate the Trident's ability to perform parallel solution phase
synthesis and to handle air-sensitive reagents such as Ti(OiPr)4, alkyl isocyanates, acid
chlorides and aryl sulfonyl chlorides.
INTRODUCTION
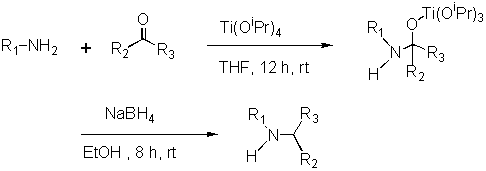
The generation of molecular diversity by parallel solution-phase synthesis is of current interest in discovery research. The Trident automated synthesizer has been designed to perform high-throughput organic synthesis under inert conditions and in a robust, reproducible manner to generate high quality libraries of small molecules. Herein, we describe a multi-step split synthesis approach for the generation of urea, amide and sulfonamide libraries in solution-phase on the Trident automated synthesizer. The sequence started with the synthesis of secondary amines by reductive amination 2 of aldehydes and ketones with primary amines using titanium(IV) isopropoxide1 and sodium borohydride. The secondary amines were then utilized for split synthesis to generate the libraries of ureas, amides and sulfonamides (Schemes 1, 2 and 3).

Scheme 1: Synthesis of Secondary Amines by Titanium(IV) Isopropoxide Mediated Reductive Amination Reaction
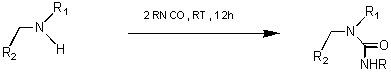
Scheme 2: Urea Synthesis by condensation of Secondary Amines with Alkyl Isocyanates
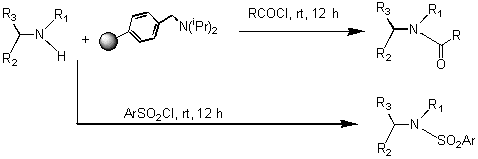
Scheme 3: Synthesis of Amides and Sulfonamides
LIBRARY CHEMISTRY
In the split synthesis approach3 for the generation of compound libraries, the products from each diversity step are used as feed-stocks for the subsequent step of the synthesis. Thus, in our synthetic scheme, we required enough quantities of secondary amines from the first step to split for the subsequent step of the synthesis.
Reductive Amination: The 48 reductive amination reactions performed on the Trident included the synthesis of 24 secondary amines in duplicate. These reactions were carried out in duplicate in order to provide enough secondary amine for the second step. These secondary amines were subsequently utilized to generate the libraries of ureas, amides and sulfonamides. The reagent matrix used 4 primary amines, 3 aldehydes and 3 ketones (Scheme 4). The reductive amination protocol1 involves two steps in one pot. The formation of an intermediate titanium complex (Scheme 1) from a carbonyl compound, amine and titanium(IV) isopropoxide was followed by reduction with sodium borohydride in ethanol. The reaction was then quenched with water and the resulting inorganic precipitate was filtered. The plan for product amine purification was centered on "catch and release" with a sulfonic acid functionalized resin.
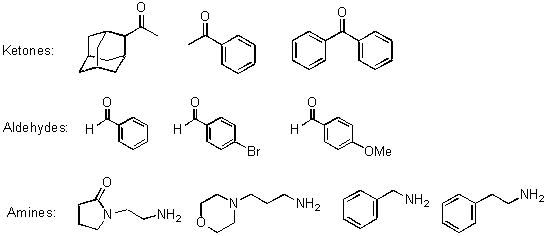
Scheme 4: Primary Amines, Aldehydes and Ketones used in Reductive Amination
Urea Library: 12 secondary amines (derived from 4 primary amines and 3 aldehydes), and 8 isocyanates (Scheme 5) were employed for the generation of the urea library. The reaction was carried out by stirring a solution of the amine and the alkyl isocyanate in DCM for 12 h at rt. Purification of the products was performed by scavenging off excess isocyanates using a resin-bound trisamine.
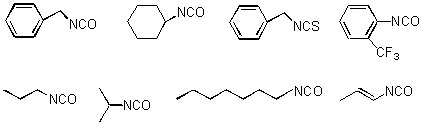
Scheme 5: Isocyanates used for Urea Library
Amide and Sulfonamide Library: The 12 secondary amines were also used for amide and sulfonamide coupling. The 96 reactions performed on Trident used 12 secondary amines, 4 acid chlorides and 4 aryl sulfonyl chlorides (Scheme 6). The synthesis was performed by stirring a mixture of the amine, the acid chloride and a resin-bound tertiary amine for 12 h at rt. The plan for purification of the product amides and sulfonamides was centered on the use of a resin-bound trisamine to scavenge excess acid/sulfonyl chloride.
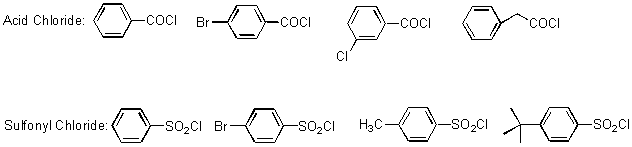
Scheme 6: Acid Chlorides and Aryl Sulfonyl Chlorides used for Amide/Sulfonamide Library
RESULTS AND DISCUSSION
The reductive amination procedure involved two steps. The titanium(IV) intermediate was allowed to form first by stirring a mixture of primary amine, carbonyl compound and titanium(IV) isopropoxide in THF at room temperature, followed by the addition of sodium borohydride in ethanol. The delivery of titanium(IV) isopropoxide proceeded smoothly demonstrating the ability of Trident to execute a synthesis containing a highly moisture-sensitive reagent. The reaction contents remained clear throughout the process and were drained to collection vials and quenched with water. The product amines were isolated and purified by using a resin-bound sulfonic acid.
Both aldehydes and ketones were reductively aminated to produce the secondary amines in good to excellent yields. The titanium(IV) mediated reaction conditions offered clean conversions affording only the desired secondary amines with no over-alkylation of the product amines observed. The reaction conditions were found suitable for reductive amination of sterically hindered and enolizable aldehydes and ketones. The purity (GC area percent) and yield of the secondary amines are shown in Figure 1. The graph gives the percentage of products formed within a defined purity and yield ranges. The results show that 92% of the products were formed in greater than 98% purity. Also, 45% of the compounds were formed in greater than 98% purity and 85% yield, and 42% of the compounds were formed in greater than 98% purity and 70% yield. Lower purity was observed in samples derived from benzophenone due to the presence of residual primary amines. These products were further purified with a resin-bound aldehyde to scavenge residual primary amines. The consistency in purity and yield for the replicate samples (two per compound) was excellent.
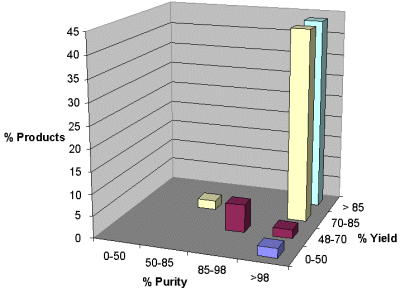
Figure 1. Population map of secondary amines as a function of purity and yield range.
The 12 secondary amines obtained from reductive amination of aldehydes were used for condensation with isocyanates to synthesize 96 ureas in duplicate. Excess isocyanate was removed from the product using a resin-bound trisamine. The purity (HPLC area percent) and yield of the ureas are shown in Figure 3. The population graph shows the percentage of products formed within a defined purity and yield ranges. The results show that 82% of the products were formed in greater than 85% yield, 43% of the products had greater than 85% purity and 39% had a purity range of 84-70%. The consistency in purity and yield for the replicate samples (two per compound) was excellent. The results again demonstrate high reproducibility in the synthesis of compounds on the Trident.
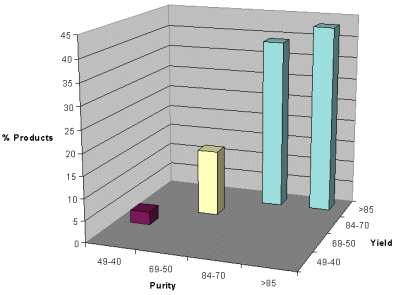
Figure 2. Population map of ureas as a function of Purity and Yield Range.
The more hindered secondary amines derived from ketones were used for coupling with acid and sulfonyl chlorides to synthesize 96 amides and sulfonamides. Polymer bound tertiary amine, PS-DIEA was used to trap hydrogen chloride. Excess acid/sulfonyl chloride was removed from the products using a resin-bound trisamine. The purity (HPLC area percent) and yield of the amides/sulfonamides are shown in Figure 3. The graph shows the percentage of products formed within a defined purity and yield ranges. The results show that 71% of the products had greater than 85% purity. Lower purity and yield were observed in samples derived from phenacetyl chloride.
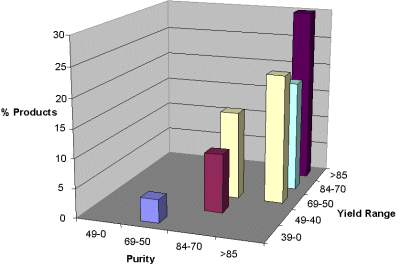
Figure 3. Population map of amides and sulfonamides as a function of purity and yield range.
SUMMARY
� 24 secondary amines were synthesized in duplicate by reductive
amination of aldehydes and ketones.
� A full capacity 192-member urea library was successfully synthesized on
the Trident Organic Synthesizer.
� A 96-member amide and sulfonamide library was synthesized on the
Trident Organic Synthesizer.
� Multiple steps were run with full automation.
� Sequence included several air and moisture sensitive reagents.
� The products were formed in high purity and yield.
� Excellent reproducibility was obtained in the cases of replicates.
REFERENCES
1. Neidigh, K. A.; Avery, M. A.; Williamson, J. S.; Bhattacharyya, S. J.
Chem. Soc. Perkin Trans 1, 1998, 2527; Bhattacharyya, S. J. Org. Chem., 1995, 60, 4928;
Bhattacharyya, S. Tetrahedron Lett., 1994, 35, 2401.
2. For a recent review see, Hutchins, R. O.; Hutchins, M. K. in Comprehensive Organic
Synthesis, eds. Trost, B. M.; Fleming, I. Pergamon, Oxford, 1991, Vol. 8, p. 25.
3. Brooking, P.; Doran, A.; Grimsey, P.; Hird, N. W.; MacLachlan, W. S.; Vimal, M.
Tetrahedron Lett., 1999, 40, 1405.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with B0010 as the message subject of your e-mail.