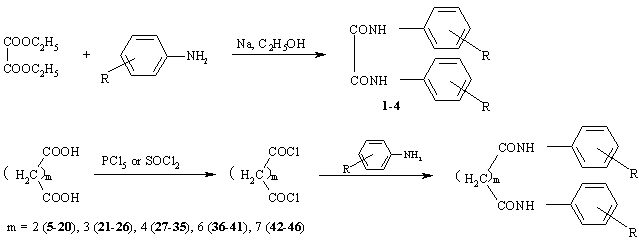
Scheme 1
[C0004]
Synthesis of N,N'-diarylalkanediamides and their antimycobacterial and antialgal activity
L. Kubicova1*, K. Waisser1, J. Kunes1, K. Kralova2, Z. Odlerova3, M. Slosarek4, J. Janota4, Z. Svoboda5
Department of Inorganic and Organic Chemistry1, Faculty of Pharmacy, Charles
University, Heyrovskeho 1205, 500 05 Hradec Kralove, Czech Republic. Tel. +420 49 5067339,
Fax +420 49 5210002, *e-mail: [email protected]
Institute of Chemistry2, Faculty of Natural Sciences, Comenius University,
Mlynska dolina CH-2, 842 15 Bratislava, Slovak Republic
Institute of Preventive and Clinic Medicine3, Limbova 14, 835 01 Bratislava,
Slovak Republic
National Institute of Public Health4, Srobarova 48, 100 42 Praha 10, Czech
Republic
Institute of Experimental Biopharmaceutics5, Heyrovskeho 1207, 500 02 Hradec
Kralove, Czech Republic
Received: 16 August 1999 / Uploaded: 21 August 1999
Abstract: A set of N,N'-diarylalkanediamides was synthesised. The compounds were tested for their antimycobacterial and antialgal activity. The antimycobacterial activity of N,N'-diarylalkanediamides depends on the lipophilicity of the respective acid. Antimycobacterially active substances were found only in the series of N,N'-diarylethanediamides and N,N'-diarylbutanediamides. Other compounds (derivatives of pentane-, hexane-, octane- and nonanediamide) were inactive against various strains of mycobacteria. The compounds inhibited growth and chlorophyll production in Chlorella vulgaris. Their relatively low antialgal activity is caused by their low aqueous solubility, and hence by a restricted passage of the inhibitor through the hydrophilic regions of thylakoid membranes.
Keywords: N,N'-diarylalkanediamides, antimycobacterial activity, antialgal activity.
Introduction
As the end of the 20th century witnesses a sharp rise in the incidence of mycobacterial infections, the development of new antimycobacterial drugs is presently of utmost importance and should proceed at a rapid pace. When exploring a possible link between antituberculous activity and the ability to form chelates with heavy metals, we prepared a set of N,N'-diarylethane- and -propanediamides, which were evaluated in vitro against Mycobacterium tuberculosis, and some of them showed significant activity [1]. Other authors have described various kinds of biological activity of alkanediamide derivatives as well. For example, some 2-methylcarbonylbutanediamides are active against M. tuberculosis [2], 2,3-diarylpentanediamides display activity against Gram-positive bacteria [3], and N,N'-substituted 2-halobutanediamides act as herbicides [4]. Raynes et al. studied the influence of the length of the connecting chain on the antimalarial activity of bisquinolines; the derivative of butanediamide was the most efficient one [5]. In our previous study [6] we found that N,N'-bis(3,4-dichlorophenyl)butanediamide effectively inhibited oxygen evolution rate (OER) in spinach chloroplasts and that this compounds interacted with the pigment-protein complexes in photosystem 2. The increase of the length of the connecting chain in the series of N,N'-bis(3,4-dichlorophenyl)alkanediamides led to the decrease of OER-inhibiting activity in spinach chloroplast [7]. The decrease in biological activity with increasing lipophilicity of the compounds is probably linked to their lowered aqueous solubility, and hence to a restricted passage of the inhibitor through the hydrophilic regions of thylakoid membranes.
This study is focused on the synthesis of a large set of N,N'-diarylalkanediamides and on the study of antimycobaterial and antialgal activity of these compounds.
Results and Discussion
N,N'-diarylalkanediamides, with the exception of N,N'-diarylethanediamides, were prepared from the corresponding anilines by treatment with the appropriate acyl chlorides in pyridine at 0░C. The reaction mixtures were allowed to stand at room temperature, and after 24 hours they were poured into water. The products were filtered off, washed with water and crystallized from ethanol. The acyl chlorides were prepared from the corresponding acids by the reaction with phosphorus pentachloride (succinyl chloride) or thionyl chloride (all other acyl chlorides). N,N'-diarylethanediamides were prepared from diethyl oxalate by the reaction with the corresponding aniline in the presence of sodium ethanolate. All syntheses are outlined in Scheme 1. The characteristic data of compounds 1--46 are given in Table 1 and Table 2.

Scheme 1
Compounds 1--46 were tested for their in vitro antimycobacterial activity against Mycobacterium tuberculosis, M. avium, M. fortuitum and M. kansasii. We found that the antimycobacterial activity of N,N'-diarylalkanediamides depends on the lipophilicity of the respective acid. Active substances were found only in the series of N,N'-diarylethanediamides and N,N'-diarylbutanediamides, but the antimycobacterial activity was mostly low. The MIC values of the active compounds are given in Table 3. All derivatives of pentane-, hexane-, octane- and nonanediamide were inactive against the above mycobacteria strains.
The inhibition of chlorophyll production in statically cultivated algae Chlorella vulgaris by selected derivatives was investigated at a constant inhibitor concentration of 75 Ámol dm-3. The antialgal activity of the compounds was generally low, and the observed inhibition of algal chlorophyll production varied in the range of 14.2 (10) to 57.9 % (40) (Table 4 and Table 5). The antialgal activity of N,N'-diarylbutanediamides was relatively low, varying in the range of 14.2 (10) to 43.6 % (15) (Table 5). The most effective inhibitor from the series of N,N'-diarylalkanediamides was N,N'-bis(4-methoxyphenyl)octanediamide (40), causing 57.9 % inhibition of chlorophyll production (Table 4). Antialgal activity of substituted N,N'-diarylalkanediamides with the same substituent R was proportional to the number of methylene groups in the connecting chain of the molecule (m = 2 -- 4, 6, 7) for derivatives with R = H and 4-Cl, respectively. For derivatives with R = 4-CH3, 4-OCH3 and 3,4-Cl2, a quasi-parabolic dependence of the inhibitory activity on m was found, with maximum inhibition for N,N'-diaryloctanediamides (m = 6; R = 4-CH3 (39) and 4-OCH3 (40)) and N,N'-bis(3,4-dichlorophenyl)hexanediamide (m = 4; 31). The relatively low biological activity of the compounds is probably a consequence of their low aqueous solubility, and hence a restricted passage of the inhibitor through the hydrophilic regions of thylakoid membranes. An efficient inhibition of photosynthetic electron transport in spinach chloroplasts by N,N'-bis(3,4-dichlorophenyl)butanediamide has been observed previously [6,7].
Tables
Table 1. Experimental data of the compounds
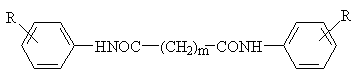 |
|||||
| Compd. | Formula (M. w.) |
m | R | M. p. (░C) Yield (%) |
IR nu(C=O) (cm-1) |
| 1 | C16H16N2O2 (268.32) |
0 | 2-CH3 | 212a) 65.2 |
1668 |
| 2 | C16H16N2O2 (268.32) |
0 | 3-CH3 | 135--137a) 59.7 |
1666 |
| 3 | C14H10N4O6 (330.26) |
0 | 4-NO2 | 357a) 61.4 |
1706 |
| 4 | C14H10Br2N2O2 (398.05) |
0 | 4-Br | 329--331a) 57.6 |
1666 |
| 5 | C16H14Br2N2O2 (426.11) |
2 | 4-Br | 281a) 92.4 |
1652 |
| 6 | C16H12Cl4N2O2 (406.10) |
2 | 3,4-Cl2 | 258--259b) 93.1b) |
1659b) |
| 7 | C16H16N2O2 (268.32) |
2 | H | 231--233a) 94.6 |
1663 |
| 8 | C18H20N2O4 (328.37) |
2 | 4-OCH3 | 256--257a) 89.5 |
1648 |
| 9 | C16H14Cl2N2O2 (337.21) |
2 | 4-Cl | 288--290 90.3 |
1652 |
| 10 | C18H20N2O2 (296.37) |
2 | 4-CH3 | 273--275 91.4 |
1655 |
| 11 | C16H14F2N2O2 (304.30) |
2 | 3-F | 206--207 88.5 |
1663 |
| 12 | C16H14F2N2O2 (304.30) |
2 | 4-F | 244--245 92.2 |
1651 |
| 13 | C16H14N4O6 (358.31) |
2 | 3-NO2 | 228--230 87.5 |
1675 |
| 14 | C20H24N2O2 (324.42) |
2 | 3,4-(CH3)2 | 231--232 85.2 |
1651 |
| 15 | C16H14Cl2N2O2 (337.21) |
2 | 3-Cl | 233--235a) 92.3 |
1668 |
| 16 | C24H32N2O2 (380.53) |
2 | 4-C4H9 | 232--234 81.0 |
1659 |
| 17 | C22H28N2O2 (352.48) |
2 | 4-isoC3H7 | 234--236 82.5 |
1656 |
| 18 | C24H32N2O2 (380.53) |
2 | 4-secC4H9 | 178--179 80.7 |
1655 |
| 19 | C20H26N4O2 (354.45) |
2 | 4-N(CH3)2 | 282.5--283.5a) 88.4 |
1645 |
| 20 | C18H14N4O2S2 (382.45) |
2 | c) | 282--285 91.5 |
1694 |
| 21 | C17H16Cl2N2O2 (351,23) |
3 | 4-Cl | 243 69.5 |
1664 |
| 22 | C19H22N2O4 (342.39) |
3 | 4-OCH3 | 224 62.2 |
1659 |
| 23 | C17H18N2O2 (282.34) |
3 | H | 225a) 84.3 |
1673 |
| 24 | C19H22N2O2 (310.40) |
3 | 4-CH3 | 221a) 54.4 |
1664 |
| 25 | C17H14Cl4N2O2 (420.12) |
3 | 3,4-Cl2 | 267b) 63.0b) |
1679b) |
| 26 | C17H16Br2N2O2 (440.13) |
3 | 4-Br | 257a) 57.5 |
1664 |
| 27 | C20H24N2O2 (324.42) |
4 | 4-CH3 | 252 67.4 |
1659 |
| 28 | C18H20N2O2 (296.37) |
4 | H | 238a) 75.0 |
1660 |
| 29 | C18H18N4O6 (386.36) |
4 | 3-NO2 | 239 58.3 |
1667 |
| 30 | C18H18Cl2N2O2 (365.26) |
4 | 3-Cl | 197 59.8 |
1663 |
| 31 | C18H16Cl4N2O2 (434.15) |
4 | 3,4-Cl2 | 277b) 58.8b) |
1670b) |
| 32 | C20H24N2O4 (356.42) |
4 | 2-OCH3 | 152 40.3 |
1659 |
| 33 | C20H24N2O4 (356.42) |
4 | 4-OCH3 | 234 64.5 |
1648 |
| 34 | C18H18Cl2N2O2 (356.26) |
4 | 4-Cl | 239 66.7 |
1656 |
| 35 | C24H28N2O6 (440.50) |
4 | 4-COOC2H5 | 217 54.0 |
1708, 1693 |
| 36 | C20H20Cl4N2O2 (462.20) |
6 | 3,4-Cl2 | 167--168b) 34.6b) |
1671b) |
| 37 | C20H22Cl2N2O2 (393.31) |
6 | 4-Cl | 197--198 81.3 |
1659 |
| 38 | C20H24N2O2 (324.42) |
6 | H | 186--188a) 57.2 |
1659 |
| 39 | C22H28N2O2 (352.48) |
6 | 4-CH3 | 224--226a) 54.7 |
1656 |
| 40 | C22H28N2O4 (384.48) |
6 | 4-OCH3 | 220--221 63.2 |
1652 |
| 41 | C20H22Br2N2O2 (482.21) |
6 | 4-Br | 253a) 53.6 |
1664 |
| 42 | C21H22Cl4N2O2 (476.23) |
7 | 3,4-Cl2 | 170--171b) 52.2b) |
1680b) |
| 43 | C21H24Cl2N2O2 (407.34) |
7 | 4-Cl | 196--197 61.4 |
1660 |
| 44 | C21H26N2O2 (338.45) |
7 | H | 180--181a) 62.7 |
1671 |
| 45 | C23H30N2O2 (366.50) |
7 | 4-CH3 | 197--198a) 61.4 |
1662 |
| 46 | C23H30N2O4 (398.50) |
7 | 4-OCH3 | 194--196 43.9 |
1655 |
| a) M. p. values from literature: Compound,
value (░C) [ref.]: 1, 209 [8]; 2, 133 [8]; 3, 359 [9]; 4,
321--322 [10]; 5, 284 [11]; 7, 230,5 [12]; 8, 256 [13]; 15,
232 [14]; 19, 277-280 [15]; 23, 223 [11]; 24, 218 [11]; 26,
256 [11]; 28, 235 [11]; 38, 186--7 [16]; 39, 219 [11]; 41, 248
[11]; 44, 186--7 [16]; 45, 198 [11]. b) The data of compounds 6, 25, 31, 36 and 42 were taken from [7]. c) The compounds is N,N'-bis(2-benzothiazolyl)butanediamide. |
|||||
Table 2. 1H NMR spectroscopic data and elemental analyses of selected compounds
| Compd. | 1H NMR delta (ppm) |
% Calc. % Found |
||
| C | H | N | ||
| 9 | 2.63 (s, 4H), 7.35--7.28 (m, 4H), 7.63--7.56 (m, 4H), 10.14 (s, 2H) | 56.99 57.24 |
4.18 4.15 |
8.31 8.38 |
| 10 | 2.21 (s, 6H), 2.60 (s, 4H), 7.11--7.03 (m, 4H), 7.48--7.40 (m, 4H), 9.89 (s, 2H) | 72.95 72.87 |
6.80 6.79 |
9.45 9.59 |
| 11 | 2.65 (s, 4H), 6.88--6.78 (m, 2H), 7.36--7.22 (m, 4H), 7.62--7.53 (m, 2H), 10.23 (s, 2H) | 63.15 62.88 |
4.64 4.49 |
9.21 9.02 |
| 12 | 2.62 (s, 4H), 7.15--7.05 (m, 4H), 7.63--7.53 (m, 4H), 10.05 (s, 2H) | 63.15 62.78 |
4.64 4,50 |
9.21 9.13 |
| 13 | 2.72 (s, 4H), 7.57 (t, J=8.2 Hz, 2H), 7.90--7.82 (m, 4H), 8.63 (t, J=2.1 Hz, 2H), 10.53 (s, 2H) | 53.63 53.22 |
3,94 3,96 |
15.64 15.78 |
| 14 | 2.12 (s, 6H), 2.14 (s, 6H), 2.58 (bs, 4H), 6.99 (d, J=8.2 Hz, 2H), 7.27 (dd, J=8.2, J=2.1 Hz, 2H), 7.34 (d, J=2.1Hz, 2H), 9.80 (s, 2H) | 74.05 73.84 |
7,46 7,37 |
8.63 8.77 |
| 16 | 0.85 (t, J=7.3 Hz, 6H), 1.33--1.18 (m, 4H), 1.55--1.42 (m, 4H), 2.52--2.45 (signal overlaped by solvent, 4H), 2.60 (s, 4H), 7.11--7.03 (m, 4H), 7.50--7.42 (m, 4H), 9.90 (s, 2H | 75.75 76.07 |
8.48 8.16 |
7.36 7.47 |
| 17 | 1.13 (d, J=6.9, 12H), 2.60 (s, 4H), 2.85--2.73 (m, 2H), 7.16--7.08 (m, 4H), 7.50--7.42 (m, 4H), 9.89 (s, 2H) | 74.97 74.93 |
8.01 8.03 |
7.97 7.97 |
| 18 | 0.72 (t, J=7.1 Hz, 6H), 1.13 (d, J=7.1 Hz, 6H), 1.57--1.40 (m, 2H), 2.60 (s, 4H), 7.11--7.05 (m, 4H), 7.50--7.44 (m, 4H), 9.91 (s, 2H) | 75.75 75.80 |
8.48 8.48 |
7.36 7.28 |
| 20 | 2.88 (s, 4H), 7.32--7.23 (m, 2H), 7.46--7.37 (m, 2H), 7.76--7.69 (m, 2H), 7.97--7.90 (m, 2H) | 56.53 56.55 |
3.69 3.87 |
14.65 14.88 |
| 21 | 1.95--1.81 (m, 2H), 2.36 (t, J=7.4 Hz, 4H), 7.35--7.29 (m, 4H), 7.64--7.57 (m, 4H), 10.05 (s, 2H) | 58.13 58.13 |
4.59 4.59 |
7.98 7.98 |
| 22 | 1.93--1.81 (m, 2H), 2.31 (t, J=7.4 Hz, 4H), 3.69 (s, 6H), 6.89--6.79 (m, 4H), 7.53--7.43 (m, 4H), 9.76 (s, 2H) | 66.65 66.93 |
6.48 6.45 |
8.18 8.24 |
| 27 | 1.63--1.55 (m, 4H),2.21 (s, 6H), 2.33--2.24 (m, 4H), 7.10--7.03 (m, 4H), 7.48--7.41 (m, 4H), 9.79 (s, 2H) | 74.05 74.21 |
7.46 7.52 |
8.63 8.66 |
| 29 | 1.70--1.60 (m, 4H), 2.43--2.33 (m, 4H), 7.57 (t, J=8.1, 2H), 7.91--7.83 (m, 4H), 8.63 (t, J=2.1 Hz, 2H), 10.41 (s, 2H) | 55.96 55.80 |
4.70 4.73 |
14.50 14.67 |
| 30 | 1.65--1.55 (m, 4H), 2.38--2.27 (m, 4H), 7.10--7.03 (m, 2H), 7.30 (t, J=8.1, 2H), 7.45--7.38 (m, 2H), 7.80 (t, J=1.9, 2H), 10.09 (s, 2H) | 59.19 59.14 |
4.97 4.97 |
7.67 7.90 |
| 32 | 1.65--1.54 (m, 4H), 2.44--2.33 (m, 4H), 3.79 (s, 6H), 6.93--6.82 (m, 2H), 7.10--6.96 (m, 4H), 7.97--7.84 (m, 2H), 9.03 (s, 2H) | 67.40 67.49 |
6.79 6.68 |
7.86 7.79 |
| 33 | 1.64--1.52 (m, 4H), 2.33--2.20 (m, 4H), 3.69 (s, 6H), 6.89--6.79 (m, 4H), 7.52--7.42 (m, 4H), 9.74 (s, 2H) | 67.40 67.62 |
6.79 6.78 |
7.86 7.88 |
| 34 | 1.65--1.55 (m, 4H), 2.37--2.26 (m, 4H), 7.35--7.29 (m, 4H), 7.63--7.56 (m, 4H), 10.03 (s, 2H) | 59.19 58.91 |
4.97 4.82 |
7.67 7.79 |
| 35 | 1.28 (t, J=7.1 Hz, 6H), 1.68--1.57 (m, 4H), 2.42--2.31 (m, 4H), 4.25 (q, J=14.2, J=7.1 Hz, 4H), 7.74--7.67 (m, 4H), 7.91--7.84 (m, 4H), 10.25 (s, 2H) | 65.44 65.70 |
6.41 6.33 |
6.36 6.17 |
| 37 | 1.36--1.25 (m, 4H), 1.64--1.49 (m, 4H), 2.27 (t, J=7.4, 4H), 7.35--7.27 (m, 4H), 7.63--7.55 (m, 4H), 10.00 (s, 2H) | 61.11 61.08 |
5.60 5.64 |
7.12 7.12 |
| 40 | 1.34--1.24 (m, 4H), 1.64--1.48 (m, 4H), 2.24 (t, J=7.4, 4H), 3.68 (s, 6H), 6.87--6.79 (m, 4H), 7.50--7.42 (m, 4H), 9.72 (s, 2H) | 68.78 68.60 |
7.24 7.15 |
7.29 7.19 |
| 43 | 1.32--1.22 (m, 6H), 1.63--1.48 (m, 4H), 2.27 (t, J=7.3 Hz, 4H), 7.34--7.28 (m, 4H), 7.62--7.55 (m, 4H), 10.00 (s, 2H) | 61.92 62.06 |
5.94 5.85 |
6.88 6.99 |
| 46 | 1.35--1.20 (m, 6H), 1.63--1.47 (m, 4H), 2.23 (t, J=7.3 Hz, 4H), 3.68 (s, 6H), 6.88--6.80 (m, 4H), 7.50--7.42 (m, 4H), 9.71 (s, 2H) | 69.32 69.61 |
7.59 7.73 |
7.03 7.07 |
Table 3. Antimycobacterial activity of the active compounds expressed as MIC (Ámol dm-3). (m = number of methylene groups in the connecting chain of the compounds)
| Compd. | m | R | MIC (Ámol dm-3) | |||
| M. tuberculosis | M. avium | M. kansasii | M. fortuitum | |||
| 3 | 0 | 4-NO2 | 37 | --a) | --a) | --a) |
| 4 | 0 | 4-Br | 4.1 | --a) | --a) | --a) |
| 6 | 2 | 3,4-Cl2 | 250 | 250 | 500 | 500 |
| 7 | 2 | H | 500 | 500 | 500 | 500 |
| 12 | 2 | 4-F | 250 | 1000 | 1000 | 1000 |
| 14 | 2 | 3,4-(CH3)2 | 500 | >1000 | >1000 | >1000 |
| 15 | 2 | 3-Cl | 500 | >1000 | >1000 | >1000 |
| 16 | 2 | 4-C4H9 | 500 | 63 | >1000 | >1000 |
| 17 | 2 | 4-isoC3H7 | >1000 | 63 | >1000 | >1000 |
| 18 | 2 | 4-secC4H9 | 500 | 63 | >1000 | >1000 |
a)
not tested
Table 4. Inhibition of chlorophyll production in Chlorella vulgaris by N,N'-diarylalkanediamides (m = number of methylene groups in the connecting chain of the compounds; concentrations of compounds were constant, 75 Ámol dm-3)
| m | Compound % of inhibition |
||||
| H | 4-CH3 | 4-OCH3 | 4-Cl | 3,4-Cl2 | |
| 2 | 7 22.8 |
10 14.2 |
8 30.8 |
9 33.2 |
6 19.8 |
| 3 | 23 41.8 |
24 26.2 |
22 41.6 |
21 34.3 |
25 28.7 |
| 4 | 28 41.0 |
27 37.7 |
33 46.0 |
34 41.4 |
31 33.5 |
| 6 | 38 44.2 |
39 41.4 |
40 57.9 |
37 45.4 |
36 22.6 |
| 7 | 44 48.5 |
45 37.9 |
46 53.9 |
43 45.5 |
42 15.1 |
Table 5. Inhibition of chlorophyll production in Chlorella vulgaris by N,N'-diarylbutanediamides (m = 2; concentrations of compounds were constant, 75 Ámol dm-3)
| Compound | R | % of inhibition | Compound | R | % of inhibition |
| 7 | H | 22.8 | 5 | 4-Br | 27.1 |
| 11 | 3-F | 25.2 | 17 | 4-isoC3H7 | 36.5 |
| 15 | 3-Cl | 43.6 | 16 | 4-C4H9 | 32.6 |
| 13 | 3-NO2 | 25.4 | 18 | 4-secC4H9 | 36.5 |
| 12 | 4-F | 35.4 | 19 | 4-N(CH3)2 | 28.8 |
| 9 | 4-Cl | 33.2 | 6 | 3,4-Cl2 | 19.8 |
| 10 | 4-CH3 | 14.2 | 14 | 3,4-(CH3)2 | 26.7 |
| 8 | 4-OCH3 | 30.8 |
Experimental
General
The melting points were determined on a Kofler block and are uncorrected. The samples for elemental analysis and biological tests were dried over P2O5 at 61 ░C and 66 Pa for 24 h. Elemental analyses were performed on a C,H,N,S analyzer (FISONS AE 1110, Milano). The IR spectra were measured in KBr on a Nicolet Impact 400 apparatus. The purity of the compounds was checked by TLC. TLC was performed using petroleum ether : ethyl acetate (1:1), and chloroform : aceton (9:1) as the mobile phases. 1H NMR spectra of new compounds were recorded for DMSO-d6 solutions at ambient temperature on a Varian Mercury-Vx BB 300 spectrometer (operating at 300 MHz). Chemical shifts were recorded as delta values in parts per million (ppm), and were indirectly referenced to tetramethylsilane via the solvent signal (2.49 for 1H).
Synthesis of acyl chlorides
Succinyl choride
Phosphorus pentachloride (328 mmol) was added to a finely powdered succinic acid (400
mmol), and the mixture was heated at 120░C for 5 hours. Phosphorus oxychloride was then
distilled off, and the crude product was purified by vacuum distillation (yield 74.5 %, b.
p. 104-105 ░C / 3.33 kPa, lit. [17] b. p. 103--104 ░C / 3.33 kPa).
Suberyl choride
Suberic acid (287 mmol) was heated with thionyl chloride (1260 mmol) at 120 ░C for 4
hours. After the removal of the excess reagent by distillation, the crude product was
purified by distillation at reduced pressure (yield 69.20 %, b. p. 145░C / 1.33 kPa, lit.
b. p. [18] 149--150 ░C / 1.60 kPa). The same protocol was used for the preparation of the
remaining acyl chlorides (yield; b. p.; b. p. [ref]): glutaryl chloride (90.7 %; 104 ░C /
2.53 kPa; 100 ░C / 2.00 kPa [19]); adipoyl chloride (81.2 %; 130--132 ░C / 2.40 kPa;
130--132 ░C / 2.40 kPa [20]); azelayl chloride (71.4 %; 162 ░C / 1.20 kPa; 165 ░C /
1.73 kPa [20]).
Synthesis of N,N'-diarylalkanediamides
N,N'-diarylethanediamides (1--4)
Sodium (1 g) was dissolved in absolute ethanol (100 ml), 3-methylaniline (300 mmol)
followed by diethyl oxalate (150 mmol) were added to the solution, and the reaction
mixture was heated at reflux for 1 hour. The crude product was filtered off, washed with
water, and crystallized from ethanol. The yields, melting points, and IR spectral data of
compounds 1--4 are given in Table 1.
Other N,N'-diarylalkanediamides
Succinyl chloride (16 mmol) was added dropwise to a stirred solution of 4-methylaniline
(32 mmol) in pyridine (20 ml) at 0░C. The reaction mixture was allowed to stand at
ambient temperature for 24 hours, and then poured into water (100 ml). The product was
filtered off and crystallized from ethanol. The remaining amides were prepared in an
analogous fashion. The yields, melting points, IR, and NMR spectral data as well as
elemental analyses are summarised in Table 1 and Table 2.
Biological assays
Antimycobacterial activity
Antimycobacterial evaluation of N,N'-diarylalkanediamides (m = 0, 3, 4, 6, 7) was carried
out in a semisynthetic liquid protein-containing Sula medium (IMUNA, Sarisske Michalany),
buffered to pH 7.2. The following mycobacterial strains were used: Mycobacterium
tuberculosis H37Rv, M. kansasii PKG8, M. avium No. 80/72 and M.
fortuitum 1021. The MICs were determined after 14 days of incubation at 37 ░C. The
compounds were added to the medium in a dimethyl sulfoxide solution. The final
concentrations were 1000; 333; 111; 37; 12.3; and 4.1 Ámol dm-3.
Antimycobacterial activity of butanediamides (m = 2) was determined in Sula semisynthetic medium (SEVAC, Prague). For evaluation of their in vitro antimycobacterial activity, the following strains were used: M. tuberculosis CNCTC My 1/47, M. kansasii CNCTC My 235/80, M. avium CNCTC My 80/72 and M. fortuitum CNCTC My 187/73 from the National Institute of Public Health, Prague. The compounds were added to the medium in dimethyl sulfoxide solutions. The final concentrations were 1000; 500; 250; 125; 62; 31; 16; 8; 4 Ámol dm-3. The minimum inhibitory concentrations were determined after incubation at 37 ░C for 21 days.
MIC was the lowest concentration of a substance (on the above-stated concentration scale), at which inhibition of the growth of mycobacteria occurred. The compound is considered as active, when its MIC is lower than 1000 Ámol dm-3.
Antialgal activity
The inhibitory effect of selected N,N'-diarylalkanediamides on algal chlorophyll (Chl)
production has been investigated in statically cultivated Chlorella vulgaris (96
hours; photoperiod 16 h light / 8 h dark; illumination: 5 000 lx; pH = 7.2; Chl content at
the beginning of cultivation: 0.5 mg dm-3) at room temperature and a constant
inhibitor concentration 75 Ámol dm-3 according to Kralova et al. [21]. Chl
content of algal suspensions was determined spectrophotometrically following its
extraction into N,N-dimethylformamide according to Inskeep and Bloom [22]. The compounds
were dissolved in dimethyl sulfoxide (DMSO) as their solubility in water was insufficient.
The antialgal activity was expressed as the percentage of inhibition of the untreated
control.
Acknowledgements: This study was supported by the Ministry of Education of the Czech Republic (CEZ J 13/98: 11600001), and by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic (grant No. 1/4013/97).
References
[1] Waisser K., Odlerova Z., Grunert R.: Pharmazie 1989, 44, 162.
[2] Dave M. P., Patel J. M., Langalia N. A., Shah S. R., Thaker K. A.: J. Indian Chem. Soc. 1985, 62, 386.
[3] Al-Arab Mohammad M., Na'was Tarek E., Abu-Yousef Imad A.: Indian J. Chem. 1990, 29B, 694.
[4] Stauffer Chemical Co.: Ger. Offen. 24 51 512 (1975).
[5] Raynes K., Galatis D., Cowman A. F., Tilley L., Deady L. W.: J. Med. Chem. 1995, 38, 204.
[6] Kubicova L., Kadlcikova J., Waisser K., Kralova K., Sersen F., Slosarek M., Janota J., Svoboda Z.: Folia Pharm. Univ. Carol. 1998, 23 Suppl., 148.
[7] Kubicova L., Kralova K., Waisser K.: Chem. Papers 1999, 53, in press.
[8] Lapkin I. I., Andrejchikev Ju. S.: Zh. Org. Khim. 1965, 1, 1212.
[9] Price Ch. C., Velzen B. H.: J. Org. Chem. 1947, 12, 386.
[10] CIBA-GEIGY: U. S. 3906033 (1975); Chem. Abstr. 1976, 84, 4633.
[11] Barnicoat C. R.: J. Chem. Soc. 1927, 2926.
[12] Kirshanov, Egorova: Zh. Obsch. Khim. 1953, 23, 1920.
[13] Ueda Mitsuru, Morishima Makoto, Kakuta Urayumi, Sugiyama Yunichi: Macromolecules 1992, 25, 6580.
[14] Sanna A., Repetto G.: Gazz. Chim. Ital. 1927, 57, 777.
[15] Chen Ku Lin, Chang Shih Tai, Chi Chieh Chang: Yao Hsuch Pao 1957, 5, 333; ref. Chem. Abstr. 1961, 55, 25862i.
[16] Hill J. W., Wallace H. C.: J. Amer. Chem. Soc. 1933, 55, 5023.
[17] Vorlaender D.: Justus Liebigs Ann. Chem. 1894, 280, 167.
[18] V. Auwers, Schmidt: Chem. Ber. 1913, 46, 479.
[19] Hoffmann H. M. R., Haase K., Geschwinder P. M.: Synthesis 1982, 3, 237.
[20] Etaix: Ann. Chim. (Paris) 1896, 9, 369.
[21] Kralova K., Sersen F., Melnik M.: J. Trace Microprobe Techn. 1998, 16, 491.
[22] Inskeep W. P., Bloom P. R.: Plant. Physiol. 1985, 77, 483.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with C0004 as the message subject of your e-mail.