[C0006]
Synthesis of Glycosyl Phosphoramidates: Novel Isosteric Analogs of Glycosyl Phosphates Thanukrishnan Kannan, Subramaniam Vinodhkumar and Duraikkannu Loganathan * Indian Institute of Technology Madras, Chennai-600 036, IndiaReceived: 26 July 1999 / Uploaded: 6 August 1999
Abstract: Several glycosyl phosphoramidates, which are novel isosteric analogs of glycosyl phosphates, were synthesized in four steps starting from the free sugars, the key step being the Staudinger reaction of fully acetylated b -D-glycosyl azide with trimethyl phosphite.
Keywords: Glycosyl phosphoramidates, Isosteric analogs, Staudinger reaction.
2. Results and Discussion
3. Table
4. References
Phosphorylation is an important and necessary reaction in living systems for their survival and function. Glycosyl phosphates play a central role in carbohydrate metabolism1. Nature employs them as glycosyl donors in the biosynthesis of oligo- and polysaccharides and glycoconjugates. N-Acetyl-a -D-glucosamine 1-phosphate, for example, is the key intermediate in the biosynthesis of N-glycoproteins. a -D-glucose 1-phosphate is formed by phosphorylase catalysed cleavage of glycogen. Synthesis of isosteric analogs of such glycosyl phosphates is a useful endeavor for the better understanding of the enzymatic pathways involved in carbohydrate metabolism. More importantly, synthesis of potent analogs that could regulate the metabolism would lead to the rational development of carbohydrate-based therapeutics.
Literature survey on the synthesis of isosteric analogs of glycosyl phosphates reveals several reports2 on the replacement of the O-P bond by a C-P bond. The synthesis of such phosphono analogs often involved multiple steps, thus resulting in low overall yield. Among the various phosphonate analogs synthesized, only b -D-fructose 2,6-bisphosphate has been evaluated for its inhibitory activity. The inhibition of rabbit liver fructose-1,6-bisphosphatase by this bisphosphonate was found to be about three orders of magnitude less effective than fructose 2,6-bisphosphate. The replacement of the oxygen atom of the phosphoester bonds with methylene groups dramatically decreases the affinity of the molecule for the binding site of the enzyme3. In view of the better electronic distribution and H-bonding capacity of -NH group, glycosyl phosphoramidates are expected to be potent isosteric inhibitors. We report herein the synthesis and characterization of several glycosyl phosphoramidates.
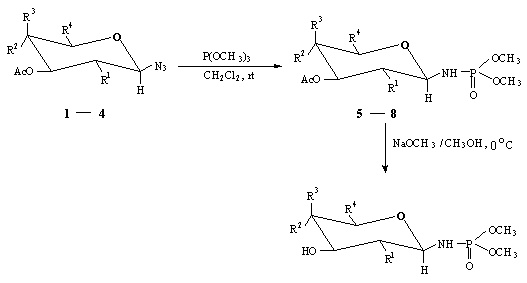
Results and DiscussionThe general methodology employed for the synthesis of glycosyl phosphoramidates utilizes the Staudinger reaction of per-O-acetylated b -D-glycosyl azides with trimethyl phosphite ( Scheme 1 ) followed by de-O-acetylation using NaOMe / MeOH. The protected glycosyl azides ( 1-4 ) were prepared following the literature procedure4 and employing benzyltriethylammonium fluoborate as the phase transfer catalyst.
In a typical procedure, reaction of 3,4,6-tri-O-acetyl �2-deoxy-2-acetamido-b -D-glucopyranosyl azide (2 ) with trimethyl phosphite in dichloromethane at room temperature for 3 hours led to the formation of a single product isolated in 93 % yield. 1H-NMR data of this product were entirely consistent with the structure of bismethoxyphosphoramidate. The b -anomeric linkage was confirmed by the appearance of a multiplet in the 1H-NMR spectrum at 4.54 ppm which on D2O exchange collapsed to a doublet of doublet with J values of 9.8 and 7.8 Hz revealing the large H1-H2 vicinal coupling and the long-range H1-P coupling respectively. 31P-NMR spectrum of this product displayed a single signal at d 7.04 ppm. The high resolution FAB mass spectrum displayed molecular ion [M+Cs]+ peak at m/z 477.1263 (calculated 477.1290 ) fully supporting the structure. De-O-acetylation of this product using NaOMe / MeOH afforded the corresponding free phosphoramidate in essentially quantitative yield and the same was fully characterized based on 1H, 13C and 31P spectral data.
The other per-O-acetylated glycosyl phosphoramidates synthesized are listed in Table 1. Considering the minimum number of steps involved and the high yields obtained, the extension of the methodology to the synthesis of phosphoramidate analogs of other biologically important glycosyl phosphates and their biological evaluation would prove to be very useful. Furthur efforts along this direction are in progress in our laboratory.
Scheme 1
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
|
R1 |
OAc |
NHAc |
OAc |
OAc |
OH |
NHAc |
OH |
OH |
R2 |
OAc |
Oac |
H |
OAc |
OH |
OH |
H |
OH |
R3 |
H |
H |
OAc |
H |
H |
H |
OH |
H |
R4 |
CH2OAc |
CH2OAc |
CH2OAc |
H |
CH2OH |
CH2OH |
CH2OH |
H |
Compound no. |
Time ( h ) |
% yield |
5 |
3 |
93 |
6 |
3 |
89 |
7 |
3 |
87 |
8 |
6 |
66 |
References
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with C0006 as the message subject of your e-mail.