Third International Electronic Conference on Synthetic Organic Chemistry (ECSOC-3), www.reprints.net/ecsoc-3.htm, September 1-30, 1999
[c0007]
Dejan Opsenicaa, Gabriela Pocsfalvib, Zorica Juranicc, Bogdan Solajad*
aInstitute of Chemistry, Technology and Metallurgy, Belgrade
bCentro Internazionale di Servizi di Spettrometria di Massa del C.N.R, Naples
cInstitute for Oncology and Radiology of Serbia, Belgrade
dFaculty of Chemistry, University of Belgrade, Studentski trg 16, P.O. Box 158, YU-11001 Belgrade, Yugoslavia
E-mail: bsolaja@chem.bg.ac.yu
Received: 29 July 1997 / Uploaded: 4 August 1997
Abstract: Cholic acid derived steroidal tetraoxanes possessing methyl ester,
carboxylic acid, primary and secondary amide termini were synthesised and their in
vitro antitumor activity against Fem-X and HeLa cell cultures was determined. IC50
range between 3.1 and 166 ![]() M. Five
out of seven tested compounds exhibited cell rounding and / or apoptic activity. Their
liquid secondary ionization (LSI) and electronspray ionization (ESI) spectra confirmed the
tetraoxane structure.
M. Five
out of seven tested compounds exhibited cell rounding and / or apoptic activity. Their
liquid secondary ionization (LSI) and electronspray ionization (ESI) spectra confirmed the
tetraoxane structure.
Keywords: Steroids, cholic acid, tetraoxanes, LSIMS, ESI, antitumor activity.
1,2,4,5-Tetraoxanes are a class of compounds usually synthesised for ring-enlargement purposes [1], and more recently, for their established antimalarial activity [2]. Activity of 1,2,4,5-tetraoxanes against Plasmodium falciparum D6 and W2 clones is very similar to that of trioxanes such as naturally occurring artemisinin, its semi-synthetic derivatives [3] and related compounds [4]. Cholestane based steroidal tetraoxanes were prepared [5] in order to explore the influence of bulky substituent(s) on 1,2,4,5-tetraoxane ring on their activity, as well as the possible influence of natural carrier. So far no report on tetraoxane antitumor activity has been published.
Here, we present the results of our further studies on steroidal tetraoxanes, the synthesis, (preliminary) structure determination and in vitro antitumor activity of some of cholic acid derived tetraoxanes [6].
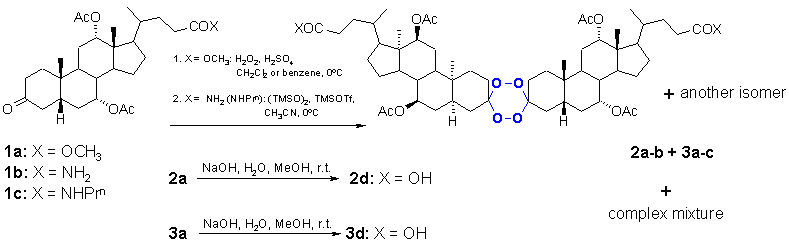
Tetraoxanes 2a (15%, 250-251 oC) and 3a (13%, mp 167-171 oC) were synthesised from parent ketone 1a (X = OCH3) using procedure given in [5], Scheme 1. The acids 2d (56%, mp 206-210 oC) and 3d (47 %, mp 189-192 oC) were obtained by alkali hydrolysis of corresponding esters. Tetraoxanes 2b (26%, mp 211-217 oC), 3b (24%, mp 196-199 oC) and 3c (20%, mp 171-174 oC) were prepared using (TMSO)2 / TMSOTf method of Jefford et al. [7]. In each case (except hydrolysis reaction) the complex mixture of products was obtained, from which the given tetraoxanes were obtained by chromatography on SiO2 column. Two isomeric methyl esters (2a and 3a, hence two acids 2d and 3d) were isolated, as well as a pair of primary amides (2b and 3b). Propyl amide 1c afforded only tetraoxane 3c. The stereochemistry of steroid substituents at 1,2,4,5-tetraoxane ring is not determined as yet (lowest energy conformations of the pair of stereoisomers is shown in Figure 1 (X = OCH3)). The 1H and 13C NMR spectra (vide infra) of each pair are almost identical (at the level of 200 MHz spectrometer), but clear distinction between tetraoxanes within each pair can be made based on the appearance of corresponding acetate methyl groups (compare 1H NMR spectra, Figure 2). In one series, the acetate methyls appear as broad singlet (at ca. 2.10 ppm), in the another, as broad singlet (at ca. 2.12 ppm) followed by another smaller broad singlet at 2.07 ppm. Tetraoxane 3c belongs to the second group. Liquid secondary ionization and electrospray ionization mass spectra of the above compounds resulted in molecular ion peaks in all of the cases. These experiments along with the LSIMS high resolution probe accurate mass measurements confirmed that we are dealing with tetraoxanes and not with hexaoxanes [8], Table 1.
The effect of tetraoxanes 2a-3d on Fem-x and HeLa cell
survival, 72 h after the agent's action is given in Table 2.
Investigated compounds expressed the dose dependent antiproliferative action toward
investigated cell lines. In order to compare the extent of the antiproliferative action
between the members of this group, IC50 were determined under exactly the same
conditions. They were similar for Fem-X and HeLa cells with exemption for 3c. This
compound inhibited selectively more melanoma then HeLa cell growth. Morphological
examination of target cells on inverted microscope showed that cytotoxic action of 3a,
2b, 3b, 2d and 3d resulted in target cell rounding and / or
cell fragmentation to apoptic bodies, typical for action of known cytostatic
cis-diamminedichloroplatinum(II), an apoptosis inducing agent. Tetraoxane 3c
inhibited HeLa cell growth without the signs of direct cell cytotoxicity up to 66 ![]() M.
M.
Figure 1. Computer generated structures of two
possible diastereomeric tetraoxanes (X = OCH3)
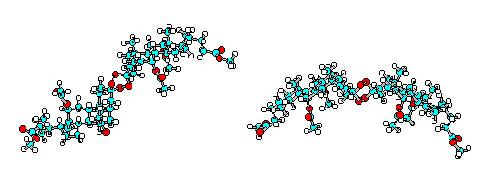
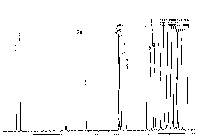
Figure 2. 1H and 13C NMR spectra of 2a and 3a, respectively.
 |
 |
 |
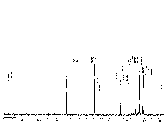 |
| 2a 1H NMR | 2a 13C NMR | 3a 1H NMR | 3a 13C NMR |
| Table 1. MS data for tetraoxanes 2a-3d | ||
| Compound | [M+H]+ [M+Na]+ | ESI Spectra were recorded on a Autospec instrument in positive ion mode using CH3CN : H2O (1:1) with 1 % AcOH as carrying solvent solution. The stock solutions of the samples were further diluted using the same buffer. The source temperature was 75 oC, and the cone voltage was set to 40 V. |
| 2a | 1041.7 (10) 1063.8 (8) | 981.6 (4), 921.7 (14), 597.0 (13), 579.0 (20), 537.4 (61), 477.3 (50), 417.3 (18), 238.0 (60), 197.0 (100), 178.2 (54), 149.0 (35) |
| 3a | 1041.7(100) 1063.8 (10) | 981.7 (15), 921.7 (23), 861.7 (4), 537.4 (70), 477.4 (82), 385.3 (18), 238.1 (16), 197.0 (26), 178.2 (23), 149.0 (14) |
LSIMS LSI mass spectra were recorded on a VG-ZAB-T instrument using ceasium ion gun. Accelerating voltage was set to 8 kV ising MNBA as matrix. Probe accurate mass measurements were performed in the presence of PEG internal callibrant at 5000 resolution. |
||
| 2d | 1035.6 (43) | 1012.6 (14), 993.6 (23), 969.6 (19), 951.6 (34), 891.5 (100), 849.5 (29), 794.0 (26), 780.0 (45), 766.1 (27), 749.1 (29), 735.0 (66%), 719.0 (34), 691.0 (36), 674.0 (47), 658.0 (42), 643.0 (53), 631.0 (58) |
| 3d | 1013.5(21) 1035.6 (100) | 1011.4 (17), 993.5 (27), 951.4 (41), 909.4 (11), 893.4 (51), 849.5 (12), 833.4 (17), 779.9 (14), 734.9 (17), 699.5 (15), 673.9 (13), 644.9 (16), 628.9 (15) |
| 2b | 1011.6 (100) | 967.7 (17), 909.6 (16), 891.6 (29), 851.6 (15), 793.6 (17), 766.4 (20), 735.5 (20), 677.5 (24), 647.5 (15), 613.2 (95), 577.4 (14), 561.4 (20) |
| 3b | 1011.6 (80) | 967.6 (6), 919.3 (10), 851.6 (6), 766.2 (14), 735.5 (10), 677.5 (11), 613.2 (100), 581.2 (9), 566.2 (11) |
| 3c | 1095.8 (100) | 1035.8 (5), 967.7 (2), 909.6 (2), 851.7 (2), 793.6 (3), 766.2 (4), 677.5 (3), 613.2 (16) |
| Table 2. IC50 ( |
||
| IC50 ( |
||
| Compound | Fem-X | HeLa |
| 2a | >29 | >29 |
| 3a | 19.5 | 20.5 |
| 2b | 3.1 | 3.7 |
| 3b | 6.2 | 6.0 |
| 3c | 94 | 166 |
| 2d | 4.2 | 5.2 |
| 3d | 7.6 | 9.2 |
[1] a) P. R. Story, D. D. Denson, C. E. Bishop, B. C. Clark Jr., J-C.
Farine, J. Am. Chem. Soc., 1968, 90, 817. b) J. R. Sanderson, P. R.
Story, K. Paul, J. Org. Chem., 1975, 40, 691.
[2] J. L. Vennerstrom, H-N. Fu, W. Y. Ellis, A. L. Ager Jr., J. K. Wood,
S. L. Andersen, L. Gerena,W. K. Milhous, J. Med. Chem., 1992, 35,
3023. b) Y. Dong, H. Matile, J. Chollet, R. Kaminsky, J. K. Wood, J. L. Vennerstrom, J.
Med. Chem., 1999, 42, 1477. c) K. Tsuchiya, Y. Hamada, A. Masuyama, M.
Nojima, Tetrahedron Lett., 1999, 40, 4077..
[3] Selected references: a) J. N. Cumming, D. Wang, S. B. Park, T. A.
Shapiro, G. H. Posner, J. Med. Chem., 1998, 41, 952. b) P. M. O'Neill, N. L.
Searie, K. J. Raynes, J. L. Maggs, S. A. Ward, R. C. Storr, B. K. Park, G. H. Posner, Tetrahedron
Lett., 1998, 39, 6065. c) T. T. Thanh Nga, C. Menage, J-P. Beque, D.
Bonnet-Delpon, J-C. Gantier, B. Pradines, J-C. Doury, T. Dinh Thac, J. Med. Chem., 1998,
41, 4101. d) G. H. Posner, M. H. Parker, J. Northorp, J. S. Elias, P. Ploypradith, S. Xie,
T. A. Shapiro, J .Med. Chem., 1999, 42, 300.
[4] a) Y. Takaya, K-i. Kurumada, Y. Takeuji, H-S. Kim, Y. Shibata, N.
Ikemoto, Y. Wataya, Y. Oshima, Tetrahedron Lett., 1998, 39, 1361. b) G. H.
Posner, H. O' Dowd, P. di Ploypradith, J. N. Cumming, S. Xie,T. A. Shapiro, J. Med.
Chem., 1998, 41, 2164. c) M. D' Ambrosio, A. Guerriero, E. Deharo, C. Debitus,
V. Munoz, F. Pietra, Helv. Chim. Acta, 1998, 81, 1285.
[5] N.M. Todorovic, M. Stefanovic, B. Tinant, J-P. Declercq, M.T. Makler,
B.A. Solaja, Steroids, 1996, 61, 688-696.
[6] The antimalarial activity of cholic acid-derived tetraoxanes will be
published soon.
[7] C. W. Jefford, A. Jaber, J. Boukouvalas, Synthesis, 1998,
391.
[8] For problems arising with mass determination of tetraoxanes and
corresponding hexaoxanes see Y. Dong, J. L. Vennerstrom, J. Org. Chem., 1998,
63, 8582.
All comments on this poster should be sent by e-mail to (mailto:ecsoc@listserv.ariz ona.edu) ecsoc@listserv.arizona.edu with C0007 as the message subject of your e-mail.