 Chart 1.
Chart 1.[D0001]
Stereoselective Synthesis of New Podophyllotoxin Derivative: 4-cyano-4-deoxy-4’-demethylepipodophyllotoxin
_______________________________________________________________________________
Zai-Xin Chen, Wei-Yong Ma and Chun-Nian Zhang
Department of Medicinal Chemistry, Shanghai Institute of Pharmaceutical Industry, Shanghai, P. R. China
Received: 13 August 1999 / Uploaded: 21 August 1999
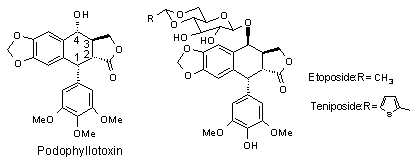
Podophyllotoxin(1), which shows high antitumor activity, is isolated from Podophyllum pelatum L. or Podophyllum emodi L.. But, because of its serious side-effect, it cannot be used as antitumor agent clinically. Its semisynthetic derivatives, Etoposide(2) and Teniposide(3), are wide-used, important anticancer drugs[1] (Chart 1). However, they have several limitations such as poor water solubility, metabolic inactivation and development of drug resistance. To overcome these limitations, many derivatives of podophyllotoxin have been synthesized in many laboratories[2]. It has been recognized that
ßsubstitution at C-4 position is necessary to the compounds with high activity. Our group has established a methodology to synthesize 4-b -substituted derivative of podophyllotoxin stereoselectively[2b]. This communication report the synthesis of 4-b -cyano-4-deoxy-4’-demethylepipodophyllotoxin, which is a important intermediate for synthesizing 4-b -carbon subtituted derivative. Chart 1.
Chart 1.
The synthetic approach started from podophyllotoxin(1). 4-demethylepipodophyllotoxin(4) was synthesized by demethylation, bromination and hydrolysis of 1, according to Kuhn’s method[3]. On the basis of our methodology, 4-b -substututed derivative of 1 or 4 can be obtained in the presence of BF3.Et2O at -15
?. We first used potassium cyanide as the nucleophilic agent. The yield was very low. HCN is very dangerous. However, in silicon agents, trimethylsilyl group often reacts as if it were a proton. So we used trimethylsilyl cyanide, a versatile silicon agent, to replace HCN. The result is very satisfying.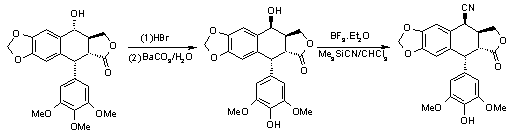 Scheme 1.
Scheme 1.
The further derivative synthesis and their biologic activities are undergoing.
References:
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with D0001 as the message subject of your e-mail.