
Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0003]
3-Dimethylaminopropenoates and Related Compounds in the Synthesis of Heterocyclic Systems and Heterocyclic Amino AcidsBranko Stanovnik
Faculty of Chemistry and Chemical Technology, University of Ljubljana,
Askerceva 5, P. O. Box 537, 1000 Ljubljana, Slovenia
E-mail: [email protected]
Received: 2
August 2000 / Uploaded: 3 August 2000
Contents
1. Introduction
2. Synthesis of 2-acylamino-3-dimethylaminopropenoates, O-substituted 2-hydroxy-3-dimethylaminpopropenoates and 2-[(2-substituted ethenyl)amino-3-dimethylaminopropenoates
3. Synthesis of heterocyclic systems
3.1 Synthesis of pyranones and fused pyranones
3.2 Synthesis of fused pyridinones
3.3 Synthesis of fused pyrimidinones
3.4 Synthesis of pyrroles
3.4.1 Substituted 3-aminopyrrole-2,4-dicarboxylates
3.4.2 Pyrrole-2-carboxylates
3.5 Synthesis of imidazolecarboxylates
3.6 Synthesis of pyrazoles
3.7 Synthesis of 1,2,4-oxadiazoles
3.8 Synthesis of aplysinopsins and azaaplysinopsins
4. Synthesis and transformations of chiral 3-dimethylaminopropenoates
4.1 A one-step synthesis of (S)-3-heteroarylalanines
4.2 Synthesis 3-heteroaryl substituted lactates
5. References and notes
1. Introduction
a -Amino and a -hydroxy acids and their derivatives play an important role in organic synthesis, especially in asymmetric synthesis as chiral synthons, chiral auxiliaries, and resolving agents [1-3].
In the course of our studies of heteroaryl substituted a -amino and a -hydroxy acids and their derivatives we have prepared easily accessible 2-acylamino-3-dimethylaminopropenoates, 2-(O-substituted hydroxy)-3-dimethylaminopropenoates, and 2-ethenylamino-3-dimethylaminopropenoates, masked a -formyl-a -amino- and a -formyl-a -hydroxy acids, and their derivatives. They have turned out to be excellent reagents for the preparaton of a variety of heterocyclic systems with an amino or hydroxy acid structural element incorporated or partially incorporated into the newly formed heterocyclic ring [4]
2. Synthesis of 2-acylamino-3-dimethylaminopropenoates, O-substituted 2-hydroxy-3-dimethylaminpopropenoates and 2-[(2-substituted ethenyl)amino-3-dimethylaminopropenoates.
Alkyl 2-acylamino-3-dimethylaminopropenoates (3) can be prepared by two methods: a) by reaction of N-acylglycine (1) with phosphorus oxychloride in N,N-dimethylformamide to afford 4-dimethylaminomethylidene-5(4H)-oxazolone (2) followed by alcoholysis in the presence of potassium carbonate to give 1, or b) by treatment of 1 with N,N-dimethylformamide dimethyl acetal or t-BuOCH(NMe2) to give 1 in one pot procedure. Similarly, alkyl O-substituted 2-hydroxyacetates (4) when treated with tert-butyloxy-bis(dimethylamino)methane to give 5. (Scheme 1).

R1 |
R2 |
Ref. |
|
3a |
Me |
Ph |
5,6 |
3b |
Et |
Ph |
7 |
3c |
Me |
Me |
8 |
3d |
Et |
Me |
9 |
3e |
Me |
CF3 |
8 |
3f |
Me |
OCH2Ph |
10 |
5a |
Me |
COPh |
11 |
5b |
Et |
COPh |
11 |
5c |
Me |
CH2Ph |
11 |
5d |
Me |
Ph |
11 |
2-[(2-Substituted ethenyl)amino]-3-dimethylaminopropenoates (9) can be prepared from compounds with an active methylene group 6 by transformation into ethoxymethylidene- or dimethylaminomethylidene derivatives 7. These are with an alkyl glycinate into 8 and further with N,N-dimethylformamide dimethyl acetal into 9. (Scheme 2).
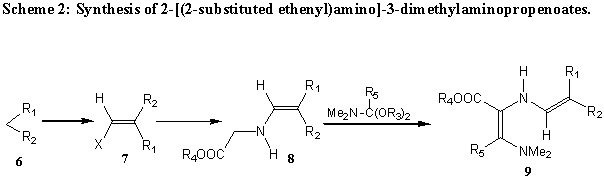
9 |
R1 |
R2 |
R3 |
R4 |
R5 |
Ref. |
a |
COOEt |
COOEt |
H |
Me |
H |
12 |
b |
COOEt |
COOEt |
H |
Et |
H |
13 |
c |
COOMe |
COOMe |
H |
Et |
H |
14 |
d |
COOEt |
COPh |
H |
Me |
H |
15 |
e |
COOEt |
COPh |
H |
Et |
H |
15 |
f |
COOEt |
COMe |
H |
Me |
H |
16 |
g |
COOMe |
COMe |
H |
Me |
H |
17 |
h |
COPh |
COPh |
H |
Et |
H |
18 |
i |
COMe |
COPh |
H |
Et |
H |
19 |
j |
COMe |
COMe |
H |
Me |
H |
20 |
k |
COOBn |
COMe |
H |
Me |
H |
17 |
l |
COOEt |
Ph |
H |
Me |
H |
21 |
m |
COOEt |
CN |
H |
Et |
H |
22,23 |
n |
COOEt |
CN |
Me |
Me |
H |
23 |
o |
COOEt |
CN |
Me |
Et |
H |
23 |
p |
COOEt |
COOEt |
H |
Me |
Me |
24 |
3. Synthesis of heterocyclic systems
2-Acylamino- and O-substituted 2-hydroxy-3-dimethylaminopropenoates and their derivatives can be applied as three carbon synthons for the synthesis of a variety of monocyclic and polycyclic heterocyclic systems, in which a -amino- or a -hydroxy acid structural element is incorporated into the heterocyclic system.
3.1 Synthesis of pyranones and fused pyranones

In the reaction of 1,3-dicarbonyl compounds 10 with 11 in the presence of acetic acid 3-acylamino-2Hpyran-2-ones 12 are formed [25]. (Scheme3).
Cyclic 1,3-dicarbonyl compounds, such as 1,3-cyclohexanedione (14) and its 5,5-dimethyl derivative (16), afford with benzyloxycarbonylamino-3-dimethylamino-propenoate (13), as an example, 5,6,7,8-tetrahydro-2H-1-benzopyran-2-ones 15 and 17, respectively. Phenol itself does not react, while resorcinol (18) and its 2-methyl derivative 20 form 2H-1-benzopyran-2-one derivatives 15 and 17 [26]. On the other hand, 1- (22) and 2-naphthol (23) are activated enough to give the corresponding 2H-naphtho[1,2-b]pyran-3-one 24 and 3H-naphtho[2,1-b]pyran-2-one 25 derivatives, respectively [27]. Similarly, 2,3-dihydroxynaphthalene (26) naphthopyranone 27 or naphthobispyranone 28. Benzyloxacarbonyl protecting group can be easily removed by catalytic transfer hydrogenation to give free amino compounds 29, 30, and 31 [10]. (Scheme 4).
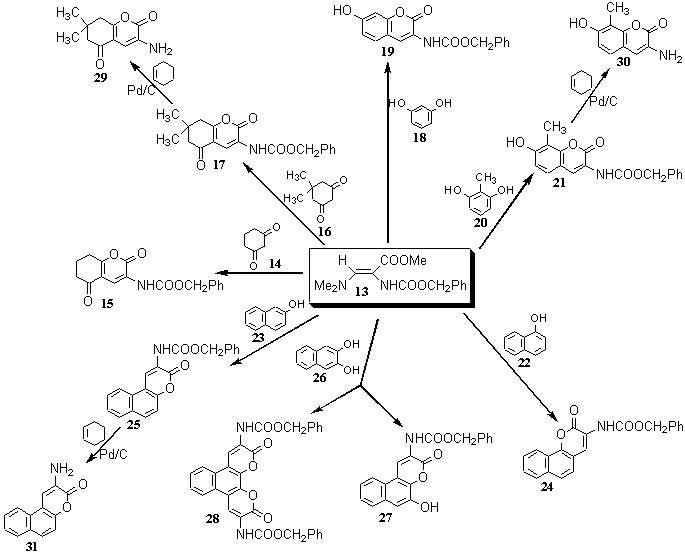

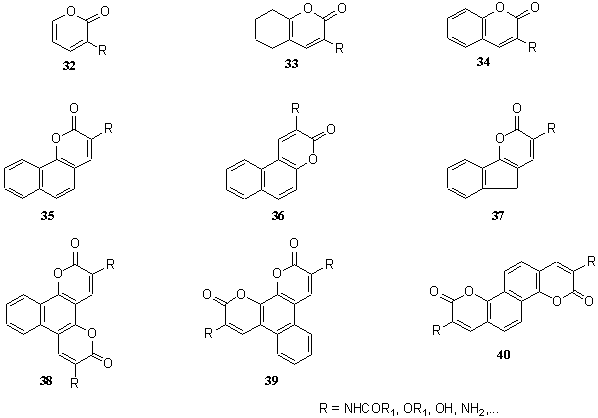
Accordingly, derivatives of the following systems have been prepared: 2H-pyran-2-ones 32 [8, 10, 25, 27], 5,6,7,8-tetrahydro-2H-benzopyran-2-one 33 [10, 15, 17, 20, 22, 27, 28], 2H-1-benzopyran-2-one 34 [8, 10, 11, 15, 17, 22, 28], 2H-naphtho[1,2-b]pyran-2-one 35 [8, 10, 11, 27, 28, 29], 3H-naphtho[2,1-b]pyran-3-one 36 [8, 10, 11, 15, 28, 30, 31], 5H-indano[1,2,-b]pyran-2-one 37 [30], 2H,6H-naphtho[1,2-b:3,4-b?]dipyran-2,6-dione 38 [30], 2H,11H-naphtho[2,1-b:3,4-b?]dipyran-2,11-dione 39 [30], and 3H,9H-naphtho[1,2-b:5,6-b?]dipyran-3,9-dione 40 [30]. (Scheme 5).
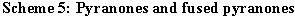
Similarly react also heterocyclic systems with a carbonyl and an adjacent metylene group as a part of the ring system 41, or their tautomeric hydroxy forms, such as pyrazole, pyridine, pyran, benzopyran, quinoline, pyridazine, tetrazolo[1,5-b]pyridazine, and pyrimidine derivatives, with 2-substituted 3-dimethylaminopropenoates 42 to yield pyranones fused to a heterocyclic system 43. (Scheme 6).
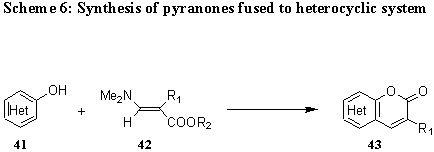
The following to heterocyclic systems fused pyranones have been prepared: 1H,6H-pyrano[ 2,3-c] pyrazole 44 [ 8,10,11,31,31,32] , 2H-pyrano[ 3,2-c] pyridine-2,5-dione 45 [ 8,10,11,15,28,31,33] , 2H,7H-pyrano[2,3-c]pyridine-2,8-dione 46, 2H,5H-pyrano[ 4,3-b] pyran-2,5-dione 47 [ 10,11,14,15,28,34] , 2H,5H-pyrano[ 3,2-c] benzopyran-2,5-dione 48 [ 8,10,11,14,15,27,28] , 2H-pyrano[ 3,2-c] quinoline-2,5-dione 49 [ 8,11,28,33] , 2H-pyrano[ 2,3-d] pyridazine-2,5-dione 50 [ 8,28,33] , 8H-pyrano[ 3,2-d] tetrazolo[ 1,5-b] pyridazin-8-one 51 [ 33] , and 7H-pyrano[ 2,3-d] pyrimidin-7-one (52). [ 8,10,11,26,28,31] . (Scheme 7)
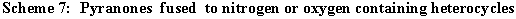
3.2 Synthesis of fused pyridinones
(Pyridinyl-2)acetic acid or its derivatives 53, such as ethyl (pyridinyl-2)acetate, (pyridinyl-2)acetonitrile, and ethyl (quinolinyl-2)acetate and 2-substituted 3-dimethylaminopropenoates 42 yield by heating in acetic acid the corresponding 4H-quinolizin-4-ones 54 and related systems. (Scheme 8).

Derivatives of the following systems have been prepared: 4H-quinolizin-4-one 55 [ 14,15,17,20,28,31,35,36] , 8H-pyrido[ 1,2-b] pyridazin-8-one 56 [ 36] , 8H-pyrido[ 1,2-c] pyrimidin-8-one 57 [ 36] , 6H-pyrido[ 1,2-a] pyrazin-6-one 58 [ 36] , and 6H-pyrido[ 1,2-a] pyrimidin-6-one 59 [ 36] . (Scheme 9).

3.3 Synthesis of fused pyrimidinones
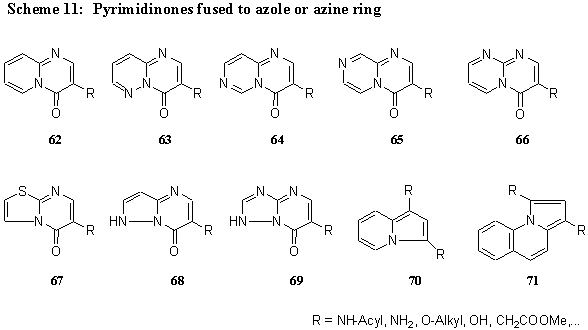
Heterocyclic a -amino compounds 60, such as 2-aminopiridines, 3-aminopyridazines, 2- and 4-aminopyrimidines, 2-aminopyrazines, 3-aminopyrazoles, 2-aminothiazoles and others, react with 2-substituted 3-dimethylaminopropenoates 42 and related compounds to form fused pyrimidinones 61 with a bridgehead nitrogen atom. (Scheme 10)

Accordingly, derivatives of the following systems have been prepared: 4H-pyrido[ 1,2-a] pyrimidin-4-one 62 [ 8,13,14,17, 20,22,31,37,38,39,40] , 4H-pyrimido[ 1,2-b] pyridazin-4-one 63 [ 17,37,38,40] , 4H-pyrimido[3,4-a]pyrimidin-4-one 64, 4H-pyrazino[ 1,2-a] pyrimidin-4-one 65 [ 37,38] , 4H-pyrimido[3,4-a]pyrimidin-4-one 66, 5H-thiazolo[ 3,2-a] pyrimidin-4-one 67 [ 8,14,17,18,20,35,37,38,41] , 7H-pyrazolo[ 1,5-a] pyrimidin-7-one 68 [ 13,31,37] , and 7H-1,2,4-thiazolo[ 1,5-a] pyrimidin-4-one 69 [ 8,37,38] , and others, such as 70 and 71. (Scheme 11).
3.4 Synthesis of pyrroles
3.4.1 Substituted 3-aminopyrrole-2,4-dicarboxylates
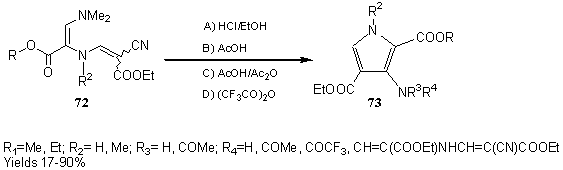
Alkyl 2-(2-alkoxycarbonyl-2-cyano-1-ethenyl)amino-3-dimethylaminopropeno-ates 72 undergo intramolecular cyclization, catalysed by acid, to give 3-aminopyrrole-2,4-dicarboxylates 73. The structure of the final product is dependent upon the reaction conditions [ 23] . (Scheme 12)
Scheme 12: Transformations of alkyl 2-(2-cyano-2-ethoxycarbonylethenyl)amino- 3-dimethylaminopropenoates. Synthesis of 3-amino-pyrrole-2,4-dicarboxylates
3.4.2 Pyrrole-2-carboxylates
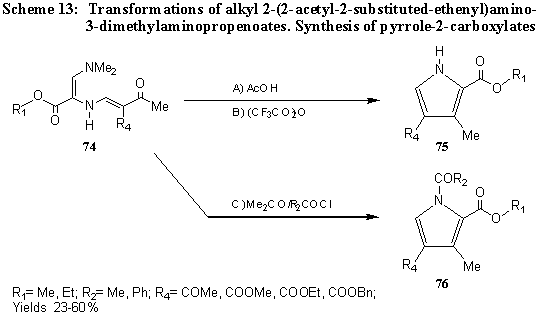
2-(2-Acetyl-2-benzoyl-1-ethenyl)amino-3-dimethylaminopropenoate and other alkyl 2-[ 2,2-bis(acyl)-1-ethenyl] amino-3-dimethylaminopropenoates and alkyl 2-(2-acyl-2-alkoxycarbonyl-1-ethenyl)amino-3-dimethylaminopropenoates 74 cyclize by heating in various solvents to give 3,4-disubstituted- 75 and 1-acyl-3,4-disubstituted pyrrole-2-carboxylates 76 [ 42,43] . (Scheme 13).
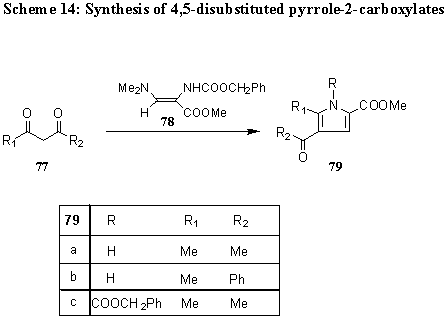
Methyl 2-(N-benzyloxycarbonyl)amino-3dimethyxlaminopropenoate 78 gives with 1,3-dicarbonyl compounds 77 5-substituted 4-acyl-1-benzyloxycarbonylpyrrole-2-carboxylate 79 [ 10] . (Scheme 14).
3.5 Synthesis of imidazole-4-carboxylates
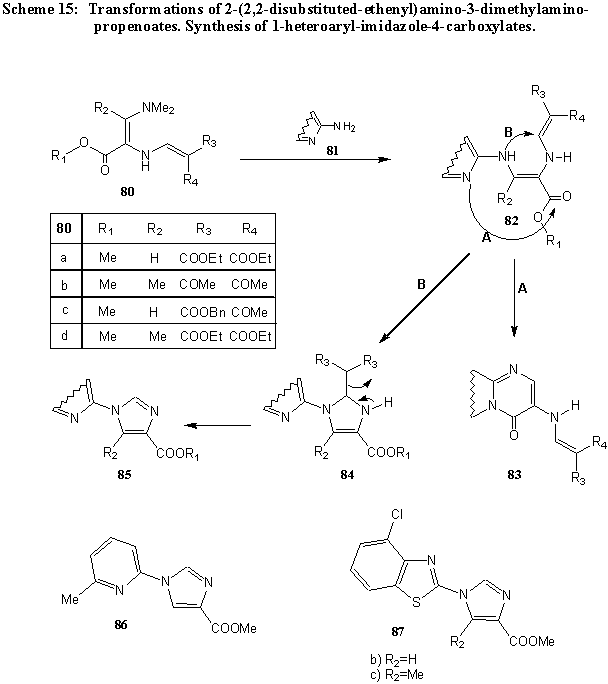
As mentioned earlier, alkyl 2-(2,2-disubstituted 1-ethenyl)amino-3-dimethylaminopropenoates 80 and heterocyclic compounds 81, with an amino group attached at a -position in respect to ring nitrogen atom, form intermediates 82, which cyclize according to path A into the corresponding azolo- and azinopyrimidinones 83. However, when these compounds are prepared from amines in which the ring nitrogen atom is sterically hindered by a substituent attached close to the ring nitrogen atom, such as in 2-amino-6-methylpyridine, 2-amino-4-chlorobenzothiazole, and its 5-methyl derivative, the reaction resulted in the formation of imidazole derivatives 85 via intermediate 84. In this manner, methyl 1-(6-methylpyridin-2-yl)-1H-imidazole-4-carboxylate (86), methyl 1-(4-chlorobenzothiazol-2-yl)-1H-imidazole-4-carboxylate (87a), and its 5-methyl derivative 87b are formed [ 12] . (Scheme 15).
3.6 Synthesis of pyrazoles
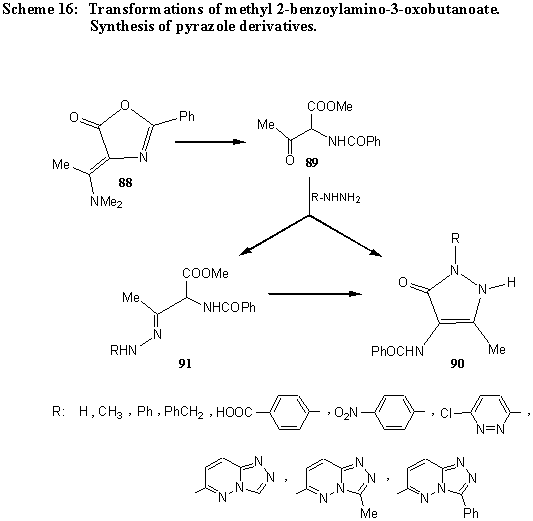
4-(1-Dimethylaminoethylidene)-2-phenyl-5(4H)-oxazolone (88), prepared from hippuric acid and N,N-dimethylacetamide as an intermediate in preparation of the corresponding propenoates, gives by hydrolysis 2-benzoylamino-2-oxobutanoate (89). In the reaction with hydrazines the corresponding 1-substituted 4-benzoylamino-3-methylpyrazol-5(2H)-ones (90) are formed. In some cases the hydrazones 91can be isolated as intermediates. [44]. (Scheme 16).
In the case of 2-(2-benzoyl-2-ethoycarbonyl-1-ethnyl)amino-3-dimethylaminopropenoate (92) two concurrent reactions take place, in which 2-(2-benzoyl-2-ethoycarbonyl-1-ethnyl)amino-3-heteroarylhydrazino)propenoates (93) and/or 4-ethoxycarbonyl-1-heteroaryl-3-phenylpyrazoles (94) are formed [45]. (Scheme 17).

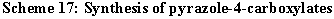
3.7 Synthesis of 1,2,4-oxadiazoles
By treatment of 2-acylamino-3-dimethylaminopropenoates (95) with nitrous acid at 0�C the corresponding oximes 96 are formed, which cyclize into 5-substituted 1,2,4-oxadiazol-3-carboxylates 97 [7,46]. (Scheme 18).
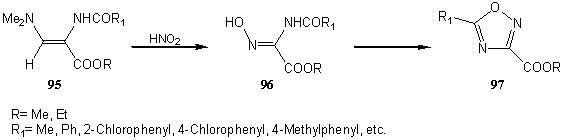

3.8. Synthesis of aplysinopsins and azaaplysinopsins
Aplysinopsins (98) and azaaplysinopsins (99) are interesting class of compounds because of their biological properties [47]. 2-(2,2-Disubstituted ethenylamino)-3-dimethylaminopropenoates can be successfully employed in the synthesis of these compounds. For example, ethyl 2-[(2-acetyl-2-methoxy(or benzyloxy)carbonylethenyl)amino]-3-dimethylaminopropenoates (100) react with indole (101) to form intermediates 102 . These, when treated with hydrazine, give intermediates 103, from which aplysinopsin (104) is formed by cyclization with urea. Alternatively, the same type of compounds can be obtained also from indole (101) and 5-dimethylaminomethylenehydantoine (106), prepared from hydantoin (105) and N,N-dimethylformamide dimethyl acetal, in good yields [48]. (Scheme 19).
Scheme 19: Application of 2-(2-acyl-2-alkoxycarbonyl-ethenyl)amino-3-dimethylamino-propenoates and 5-dimethylaminomethylenehydantoins in the synthesis of aplysinopsins
4. Synthesis and transformations of chiral 3-dimethylaminopropenoates
Chiral analogs of 3-dimethylaminopropenoates (110) were prepared from tetrahydrofuran-2-ones (109a,b) and pyrrolidin-2-ones (109c-e) by treatment with tert-butoxybis(dimethylamino)methane, respectively [49-51]. (Scheme 20).
Scheme 20: Synthesis of chiral 3-dimethylaminopropenoates
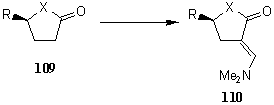
Compound |
R |
X |
Yield |
110a |
COOMe |
O |
58 |
110b |
CH2COOPh |
O |
43 |
110c |
COOMe |
N-COPh |
74 |
110d |
COOMe |
N-Boc-t |
87 |
110e |
PhCOOCH2 |
N-COPh |
71 |
4.1 A one-step synthesis of (S)-3-heteroarylalanines
(S)-1-Benzoyl-3-[(E)-dimethylaminomethylidene]-5-methoxycarbonylpyrrolidin-2-one (109c) was transformed with 1,3-dinucleophiles, such as 2-(pyridinyl-2)acetates and 1,3-dicarbonyl compounds into the corresponding quinolizinyl- (111) and 2-oxo-2H-pyranyl-3 substituted alanine esters (112) in 50-90 % yield [52]. (Scheme 21).
Scheme 21: Synthesis of (S)-3-heteroarylalanines

4.2 Synthesis of 3-heteroaryl substituted lactates
Analogously, 3-heteroaryl substituted lactic acid derivatives were prepared by ring switching methodology in a one-step transformation from (S)-3-[(E)-dimethyl- aminomethylidene]-5-methoxycarbonyltetrahydrofuran-2-one (110a) by treatment with 1,3-dinucleophiles. In this manner, 3-quinolizinyl-3- (113), 4-oxo-4H-pyrido[1,2-a]pyrmidinyl-3- (114), and 3-(2-oxo-2H-pyranyl-3)lactic acid derivatives (115) were obtained [53, 54]. (Scheme 22).
Scheme 22: Synthesis of 3-heteroaryl substituted lactates

Acknowledgment.
I would like to take this opportunity to express my sincere thanks and gratitude to my coworkers and students, especially to Docent Dr. Jurij Svete, Drs. Z. Cadez, A. Copar, S. Golic Grdadolnik, A. Hvala, M. Kmetic, M. Malesic, B. Ornik, L. Pizzioli, L. Selic, G. Sorsak, S. Strah, J. Smodis, J. Tihi, M. Skof, R. Toplak, and Ph. D. students U. Bratusek, L. Jukic, S. Recnik, C. Turk, and others; all their names are included in the references. Without their enthusiastic, creative and hard work this lecture would not have been possible. My thanks are due also to Professor L. Golic and Dr. A. Golobic for X-ray structure analyses.
References and Notes
[1] R. M. Williams, Synthesis of Optically Active a -Amino Acids, Pergamon Press. Oxford 1989.
[2] a) R. O. Duthaler, Tetrahedron 50, 1539 (1994); b) I. Wagner and H. Musso, Angew. Chem. 95, 827 (1983); c) U. Schmidt, A. Lieberknecht, and J. Wild, Synthesis 1159 (1988).
[3] J. Mulzer, Basic Principles of EPC Synthesis in Stereoselectie Synthesis, Methods of Organic Chemistry (Houben-Weyl), 4th ed., Vol. 1, G. Helmchen, ed., Thieme Vellag, Stuttgart, 1996, pp.75-146.
[4] For reviews see: a) B. Stanovnik, Methyl 2-Benzoylamino-3-dimethylamino-propenoate in the Synthesis of Heterocyclic Systems in Progress in Heterocyclic Chemistry, Vol. 5, H. Suschitzky and E. F.V. Scriven, eds., Pergamon Press, Oxford, 1993, pp. 34-53; b) B.Stanovnik, Molecules 1, 123 (1996)
[5] B. Stanovnik, J. Svete, M. Tisler, L. Zorz, A. Hvala, and I. Simonic, Heterocycles 27, 903 (1988).
[6] Japan kokai 75 58.063; Chem. Abstr. 83, P193075y (1975).
[7] M. Kmetic and B. Stanovnik,
J. Heterocyclic Chem. 32, 1563 (1995).[8] L. Kralj, A. Hvala, J. Svete, L. Golic, and B. Stanovnik, J. Heterocyclic Chem. 34, 247 (1997).
[9] L. Pizzioli, J, Svete, and B. Stanovnik, to be published.
[10] a) R. Toplak, J. Svete, B. Stanovnik, and S.Golic Grdadolnik
J. Heterocyclic Chem. 36, 225 (1999); b) S. Recnik, R. Toplak, J. Svete, L. Pizzioli, and B. Stanovnik, J. Heterocyclic Chem., submitted for publication.[11] J. Smodis and B. Stanovnik, Tetrahedron 54, 9799 (1998).
[12} L. Selic and B. Stanovnik,
J. Heterocyclic Chem. 35, 1527 (1998).[13] G. Sorsak, A. Sinur, L. Golic, and B. Stanovnik, J. Heterocyclic Chem. 32, 921 (1995).
[14] G. Sorsak, S. Golic Grdadolnik, and B. Stanovnik,
ACH-Models in Chemistry, 135, 613 (1998).[15] S. Strah, S. Golic Grdadolnik, and B. Stanovnik,
J. Heterocyclic Chem. 34, 263 (1997).[16] R. Toplak, M. Zucchiati, S. Golic Grdadolnik, and B. Stanovnik,
Heterocycles 50, 853 (1999).[17] L. Selic, S. Golic Grdadolnik, and B. Stanovnik,
Helv. Chim. Acta 80, 2418 (1997).[18] G. Sorsak, S. Golic Grdadolnik, and B. Stanovnik,
J. Heterocyclic Chem. 35, 1275 (1998).[19] M. Malesic, A. Krbavcic, A. Golobic, L. Golic, and B. Stanovnik,
J. Heterocyclic Chem. 34, 1757 (1997).[20] L. Selic and B. Stanovnik,
J. Heterocyclic Chem. 34, 813 (1997).[21] L. Selic and . Stanovnik, to be publi
shed.[22] L. Selic, S. Golic Grdadolnik, and B. Stanovnik,
Heterocycles 49, 133 (1998).[23] L. Selic and B. Stanovnik,
Helv. Chim. Acta 81, 1634 (1998)[24] L. Selic, S. Golic Grdadolnik, and B. Stanovnik,
Heterocycles 45, 2349 (1997).[25] J. Svete, Z. Cadez, B. Stanovnik, and M. Tisler, Synthesis 70 (1990).
[26] B. Stanovnik, J. Svete, and M. Tisler, J. Heterocyclic Chem. 26, 1273 (1989).
[27] B. Ornik, Z. Cadez, B. Stanovnik, and M. Tisler, J. Heterocyclic Chem. 27, 1021 (1990).
[28] R. Toplak, L.Selic, G. Sorsak, and B. Stanovnik, Heterocycles 45, 555 (1997).
[29] B. Ornik, B. Stanovnik, and M.Tisler, J.Heterocyclic Chem. 29, 1241 (1992).
[30] B.Ornik, B. Stanovnik, and M. Tisler, J. Heterocyclic Chem. 29, 831 (1992).
[31] M. Kusar, J. Svete, and B. Stanovnik, J. Heterocyclic Chem. 33, 1041 (1996).
[ 32] B. Stanovnik, J. Svete, and M. Tisler, J. Heterocyclic Chem. 26, 1273 (1989).
[ 33] M. Kmetic, B. Stanovnik, M. Tisler, and T. Kappe, J. Heterocyclic Chem. 35, 1331 (1993).
[ 34] B. Stanovnik, L. Golic, P. Kmecl, B. Ornik, J. Svete, and M. Tisler, J. Heterocyclic Chem. 28, 1961 (1991).
[ 36] A. Copar, B. Stanovnik, and M. Tisler, Bull. Soc. Chim. Belg. 100, 533 (1991).
[ 37] B. Stanovnik, H. van de Bovenkamp, J. Svete, A. Hvala, I. Simonic, and M. Tisler, J. Heterocyclic Chem. 27, 359 (1990).
[ 38] R. Toplak, J. Svete,S. Golic Grdadolnik, and B. Stanovnik, Coll. Czech Chem. Commun. 64, 177 (1999).
[ 39] S. Strah, A. Golobic, L. Golic, and B. Stanovnik, J. Heterocyclic Chem. 34, 1511 (1997).
[ 40] L. Selic, S. Strah, R. Toplak, and B. Stanovnik, Heterocycles 47, 1017 (1998).
[ 41] M. Malesic, A. Krbavcic, and B. Stanovnik, J. Heterocyclic Chem. 34, 813 (1997).
[ 42] M. Malesic, A. Krbavcic, A. Golobic, L. Golic, and B. Stanovnik, J. Heterocyclic Chem. 34, 1757 (1997).
[ 43] L. Selic and B. Stanovnik, Synthesis 479 (1999).
[ 44] U. Bratusek, A. Hvala, and B. Stanovnik, J. Heterocyclic Chem. 35, 1281 (1998).
[45] S. Strah and B. Stanovnik, J. Heterocyclic Chem. 34, 1629 (1997).
[46] M. Kmetic and B. Stanovnik
, J. Heterocyclic Chem. 34, 1705 (1997).[47] J. M.Chezal, G. Delmas, S. Mavel, H. Elakmaoui, J. Metin, A. Diez, Y. Blache, A. Gueiffier, M. Ruboralta, J. C. Teulade, and O. Chavignon, J. Org. Chem. 62, 4085 (1997).
[48] L. Selic, R. Jakse, K. Lampic, L. Golic, S. Golic Grdadolnik, and B. Stanovnik, Helv. Chim. Acta 83 (2000), in print.
[49] M. Skof, J. Svete, B. Stanovnik, L. Golic, S. Golic Grdadolnik, and L. Selic, Hlv. Chim. Acta 81, 2332 (1998).
[50] M. Skof, J. Svete, M. Kmetic, B. Stanovnik, and S. Golic Grdadolnik, Eur. J. Org. Chem. 1999, 1581.
[51] M. Skof, J. Svete, and B. Stanovnik, Heterocycles 53, 339 (2000).
[52] M. Skof, J. Svete, and B. Stanovnik, Heterocycles 51, 1051 (1999).
[53] M. Skof, J. Svete, and B. Stanovnik, Heterocycles 52, 845 (2000).
[54] For a review about the synthesis and transformations of 3-dimethylaminopropenoates and related compounds see: B. Stanovnik and J. Svete, Synlett 2000, 1077.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0003 as the message subject of your e-mail.