Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
Preparation of new enantiopure atropisomeric b-aminoalcohols and their use in asymmetric catalysis: enantioselective addition of diethylzinc to aromatic aldehydes.
Tommaso Mecca, Stefano Superchi and Carlo Rosini*
Dipartimento di Chimica, Università degli Studi della Basilicata, Via N. Sauro 85, 85100 Potenza, Italy.
Tel. +39 (0)971 202241, Fax +39 (0)971 202223, E-mail:
[email protected],
http://www.unibas.it
Received: 23 July 2000 / Uploaded: 30 July 2000
Abstract: A new class of enantiopure b-aminoalcohols having 1,1’-binaphthyl skeleton, and chiral only by atropisomerism, has been prepared and tested as catalytic precursors in the asymmetric addition of diethylzinc to aromatic aldehydes. The aldehydes were quickly and cleanly (99% yield) transformed in the corresponding alcohols with ee up to 87%.
Keywords: b-aminoalcohols, atropisomerism, 1,1’-binaphthyls, diethylzinc.
![]() Introduction
Introduction
![]() Results and Discussion
Results and Discussion
![]() Conclusions
Conclusions
![]() References and Notes
References and Notes
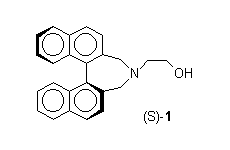
Enantiopure natural1 and synthetic2 aminoalcohols have found widespread application in asymmetric synthesis. In general these systems owe their chirality to the presence of stereogenic carbon atoms, and only recently aminoalcohols having either planar3 or atropisomeric4 chirality started to find a systematic investigation. So far the only report concerning atropisomeric b-aminoalcohols is due to Noyori,5 who described the use of aminoalcohol (S)-1 in the enantioselective addition of diethylzinc to benzaldehyde2b,6, leading to the corresponding (S)-1-phenyl-1-propanol in 49% ee.

Compounds (S)-2a-c were obtained by reacting (S)-3 with the suitable aminoalcoholic precursors as reported in Scheme
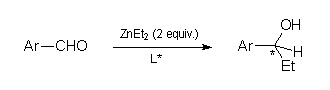
|
|
Ar | L* a | Time | Temp.(°C) | Yield (%)b | ee %.c (C.A.)d |
| 1 | Ph | (S)-2a | 14 h | 20 | 97e | 64 (S) |
| 2 | Ph | (S)-2b | 30 min | 25 | 99 | 87 (S) |
| 3 | Ph | (S)-2c | 5 h | 20 | 99 | 81 (S) |
| 4 | Ph | (S)-2c | 16 h | 0 | 99 | 78 (S) |
| 5 | Ph | (S)-2c f | 16 h | 20 | 99 | 75 (S) |
| 6 | p-CH3OC6H4 | (S)-2b | 90 min | 25 | 99 | 80 (S) |
| 7 | p-CNC6H4 | (S)-2b | 10 min | 25 | 99 | 84g (S)h |
a) 8% of aminoalcohol was used. b) Chromatographic (GLC) yield. No traces of benzylalcohol detected. c) Determined by HPLC on Chiralcel OD. d) Determined by elution order on Chiralcel OD.6c e) About 1% of benzylalcohol was detected. f) 3% of aminoalcohol was used. g) Determined by HPLC on Chiralcel OJ. h) Determined by comparison of optical rotation with literature value.11
The results collected in the Table can be summarized
as follows:
(1) In the presence of catalytic amounts of compounds (S)-2a-c
diethylzinc smoothly and cleanly adds to benzaldehyde providing 1-phenyl-1-propanol
in high chemical yield: the aldehyde is entirely converted and not even
traces of benzylalcohol (a byproduct of this reaction) can be found.
(2) (S) chirality of the binaphthyl compound always induces the
prevailing formation of the (S)- alcohol. (3) Reduction of
the amount of the ligand from 8 to 3% determines only a small reduction
of enantioselectivity (from 81% to 75%) (compare runs 3 and 5). (4)
The reduction of temperature (from 20°C to 0°C) slows down the
reaction but does not enhance the enantioselectivity (compare runs 3 and
4). (5) It is really interesting to note that simply acting on the
nature of the groups R we were able to increase the enantioselectivity
from 49% (Noyori value with (S)-15),
to 64% [(S)-2a], to 81% [(S)-2c], to 87% [(S)-2b].
In other words, making suitable structural modification (without inserting
further elements of chirality) on the original Noyori’s ligand (S)-1
we showed that high values of enantioselectivity can be obtained also using
catalysts having only atropisomeric chirality and which do not possess
any stereogenic carbon atom. Previous experimental6
and theoretical7 investigations showed that
the absolute configuration of C(O) primarily determines the stereochemical
outcome of the b-aminoalcohol catalyzed reaction.
In the present case the chiral environment created by the 1,1’-binaphthyl
nucleus is transmitted through all the molecule and affects the events
occurring at the N-Zn-O moiety, where all the steps of the enantioselective
formation of the alcohol take place. The transmission of chirality from
the binaphthyl moiety to the C(O) is demonstrated by the diastereotopicity
(revealed by
1H NMR chemical shifts) of the R substituents on
this carbon. As a consequence, even if this carbon atom is not stereogenic,
it can be defined as chirotopic, following the Mislow and Siegel definition.12
The concept of chirotopicity has been recently exploited by Knochel et
al.13 to prepare a new class of chiral ligand
for asymmetric synthesis. The possibility of this long range control of
chirality depends on the nature of the substituents at the C(O) atom: it
is low for R = H, higher for R = Me, and reaches a maximum value for R
= Ph. In the latter case the presence of the “magic” diphenylhydroxymethyl
group allows the maximum enantioselectivity thus confirming the efficiency
of this achiral moiety in asymmetric catalysis.10
The most efficient ligand (S)-2b was used in the enantioselective
addition of ZnEt2 to p-substituted aryl aldehydes with
either electrondonating (-OCH3) or electronwithdrawing (-CN)
groups. The reaction for p-methoxybenzaldehyde is slower (90 min)
than for benzaldehyde (30 min), while a very quick reaction (less than
10 min!) is observed for p-cyanobenzaldehyde: this can be clearly
related to the more electrophilic character of the carbonyl group of p-cyanobenzaldehyde
with respect to the p-methoxybenzaldehyde.14
By contrast the ee of the product is independent of the nature of the substrate.
This result seems to contradict a recent report4b
where the enantioselectivity increases with reactivity, but is in complete
agreement with other investigations6 reporting
that the enantioselectivity is mainly determined by steric factors.
We have clearly demonstrated that enantiopure atropisomeric aminoalcohols having the structure of (S)-2 can act as efficient promoter of the enantioselective addition of ZnEt2 to aryl aldehydes. This result is important from a practical point of view (a new class of efficient promoters is available) but also from a more speculative point of view: these compounds owe their chirality only to the atropisomerism of the binaphthyl nucleus and do not have any stereogenic carbon atom. It is interesting to note that this investigation fully confirms the theoretical analysis of Goldfuss and Houk7 about the need of substituents at C(O) in order to achieve higher enantioselectivity. We are convinced that the present experimental results may stimulate further investigation aimed at clarifying the correlation between structure and catalytic activity of aminoalcohol precatalysts. Work is now in progress to study also the efficiency of compounds (S)-2a-c in other asymmetric reactions.
Acknowledgements: Financial support from MURST (Roma) and Università della Basilicata (Potenza) is gratefully acknowledged.
[1] Wynberg, H. Topics Stereochem.1986,
16,
87.
[2] (a) Ager, D. J.; Prakash, I.; Schaad, D.
R. Chem. Rev.
1998, 96, 385; (b)
Fache, F.; Schulz, E.; Tommasino, M. L.; Lemaire, M. Chem. Rev.
2000,
100,
2159.
[3] (a) Bolm, C.; Muniz-Fernandez, K.; Seger,
A.; Raabe, G. Synlett. 1997, 1051. (b) Bolm, C.; Muniz-Fernandez,
K.; Seger, A.; Raabe, G.; Gunther , K.; J. Org. Chem.
1998,
63,
7860. (c) Bolm, C.; Muniz, K. Chem. Commun.
1999,
1295. (d) Bolm, C. Muniz, K. Chem. Soc. Rev.
1999,
28,
51. (e) Patti, A.; Nicolosi, G.; Howell, J. A. S.; Humphries, K.
Tetrahedron:
Asymmetry 1998, 9, 4381.
(f) Dosa, P. I.; Ruble, J. C.; Fu, G. C. J. Org. Chem.
1997,
62,
2665.
[4] (a) Vyskocil, S.; Smircina, M.; Hamas,
V.; Polasek, M.; Kocovsky, P. J. Org. Chem.
1998,
63,
7738. (b) Zhang, H.; Xue, F.; Mak, T. C. W.; Chan, K. S. J.
Org.
Chem.
1996,
61,
8002.
[5] Noyori, R.; Suga, S.; Kawai, K.; Okada,
S.; Kitamura, M.; Oguni, N.; Hagashi, M.; Kaneko, T.; Matsuda, Y. J.
Organomet.
Chem. 1990, 382,
19.
[6] (a) Noyori, R.; Kitamura, M. Angew.
Chem.
Int.
Ed.
Engl.
1991,
30,
49. (b) Soai, K.; Niwa S.; Chem.
Rev. 1992,
92,
833. (c) Dai, W.; Zhu, H.; Hao, X.
Tetrahedron:
Asymmetry
2000,
11,
2315 and references therein.
[7] Goldfuss, B.; Houk, K. N. J.
Org.
Chem.
1998,
63,
8998.
[8] Saudan, L. A.; Bernardinelli, G.; Kundig,
E. P. Synlett.
2000, 483, and references therein.
[9] (a) Gingras, M.; Dubois, F. Tetrahedron
Lett.
1999, 40, 1309. (b) Xiao, D.; Zhang, Z.;
Zhang, X. Org. Lett. 1999, 1, 1679.
[10] Braun, M. Angew. Chem.
Int.
Ed.
Engl.
1996,
35,
519.
[11] Williams, D. R.; Fromhold, M. G. Synlett.1997,
523.
[12] Mislow, K.; Siegel, J. J.
Am.
Chem.
Soc.
1984,
106,
3319.
[13] Graf, C.; Mulan, C.; Knochel, P. Angew,
Chem.
Int.
Ed.
Engl.
1998,
37,
3014.
[14] Corey, E. J.; Yuen, P.; Hannon, F. J.;
Wierda, D. A. J. Org. Chem. 1990, 55,
784.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0005 as the message subject of your e-mail.