 |
Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0007]
|
|
|
|
|
|
Received: 24 July 2000 / Uploaded: 29 July 2000
INTRODUCTION
RESULTS
REFERENCES
COMMENTS
Oxazolidine moieties are widely recognized as very useful chiral auxiliaries
in asymmetric reactions,1 and chiral epoxides have been frequently
involved in the design of strategies for the achievement of stereocontrol.2
However, only few examples reported in literature,3 combine
the influence in the same substrate of both epoxide and oxazolidine
moieties, although in any case related with intramolecular reactions.
As a part of our ongoing research program aimed at the synthesis
of biologically relevant hydroxylated amino acids,4 we wish
to report an easy strategy which allows the stereoselective preparation
of 4,5-disubstituted oxazolidinones 5, based on the use of the homochiral
epoxy oxazolidines derived from L-Serine, which could be precursors of
b,g-dihydroxy-a-amino
acids (Scheme 1).
 |
Scheme 1
Alkenyl oxazolidines 2a-d were obtained starting from Garner´s
protected aldehyde 1a (R = tert-Bu) and its N-Cbz
analogue 1b (R = Bn)5 by using the Wittig olefination
standard procedure.6 Next, epoxidation of each olefin by treatment
with MCPBA in dichloromethane, gave the corresponding threo 3a-d
and erythro 4a-c, N-protected a-amino
epoxides, with excellent stereoselectivities.7 The
threo
diastereomer
was always favoured8 (in the epoxidation of
2d, the erythro
isomer 4d, was not detected) and the resulting mixtures were conveniently
separated by conventional column chromatography
(Scheme 2).
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 2
Addition of p-TSA to a solution of the threo amino epoxides 3a-d
in anhydrous methanol at room temperature, afforded the corresponding cyclic
carbamates 5a (R´ = H) and 5b (R´ = Me). According
to the literature,9 tert-butyl carbamates 3a,
3c
reacted faster and with higher yields than their benzyl analogues
3b,
3d
and in these cases, the oxazolidinones 5 were isolated with good
to excellent yields (Scheme 3).
 |
|
|
|
|
5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scheme 3
The key-step in the asymmetric synthesis of the oxazolidinones 5
is a fully regio- and stereoselective cyclocarbamation process through
an intramolecular nucleophilic attack of the carbamate moiety on the C-a
of
the protonated epoxide under acidic conditions (Scheme 4). Such participation
of a carbamate moiety had been observed in intramolecular epoxide opening
under acidic conditions.9,10 To the best of our knowledge this
is the first example of an intramolecular nucleophilic epoxide opening
reaction in 4-oxiranyl-oxazolidines.
 |
Scheme 4
The good results obtained in the case of compounds 3a-d, suggested
a similar study with the erythro N,O-protected epoxide
4c.
However, when compound 4c was submitted under the same conditions,
failed to give the expected cyclic product 5, and compound
6
(Figure
1) was isolated, as a result of an intermolecular reaction with the methanol
used as solvent .
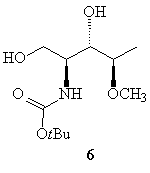 |
Figure 1
Moreover, theoretical calculations have been performed in order to explain
the faster intramolecular rearrangement towards the intermolecular displacement
in the case of the threo compounds 3. Calculations were carried
out using Gaussian94.11 Geometries were fully optimized by ab
initio calculations using restricted Hartree-Fock level of theory with
6-31G* basis sets. The structures were characterized as a minimun by a
frecuency calculation and the RHF/6-31G* energies were corrected for unscaled
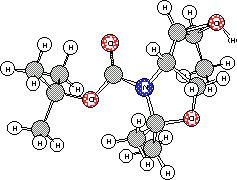
zero point energies. The optimized structures are shown in Figure 2.
The different behaviour between 4c and 3c could be explained
by the larger stability of the protonated epoxide 4c in comparison
with the protonated epoxide 3c ( relative
stability of 14.59 Kcal/mol). Apparently, one reason for the intermolecular
nucleophilic attack of the methanol in the case of the erythro compound
4c
is the formation of an intramolecular hydrogen bonding between the
carbonylic oxygen atom of tert-butoxy carbonyl group and the hydrogen
of the protonated oxygen atom of the epoxide group (interatomic distance
C=O.......H-O , 1.498 Aº ). This hydrogen bonding unables
the carbonyl group to act as a nucleophile.
The outcome of the reaction in the case of the threo epoxide
3c
could be explained by the model shown in Figure 2. In this case the
absence of an intramolecular hydrogen bond places the N-Boc group
in a such conformation that the nucleophilic attack by the carbonyl oxygen
atom is highly favoured.
The natural bond population (NBO) analysis on the protonated epoxide
3c
allows
us to understand the total regioselection affording oxazolidinones
5a,b
through a 5-exo mode cyclization. This calculations are in agreement with
larger positive charge in C-a position ( C-a
is 0.17 and C-b is 0.16) leading to the 5-exo
closure pathway. Similar calculations performed on the protonated epoxide
4c revealed that the positive charge is larger on the C-b
position ( C-a 0.12 and C-b
0.15) and the intermolecular nucleophilic attack occurs on the distal oxiranyl
carbon atom (C-b) according to the literature.1a,
2
 |
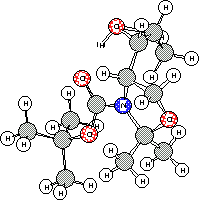 |
Protonated epoxide 3c Protonated epoxide 4c
Figure 2
CONCLUSIONS
In summary, treatment of threo amino epoxides 3a-d, with catalytic
p-TSA
in anhydrous methanol led to a highly regio- and stereoselective intramolecular
epoxide opening reaction, affording cis-oxazolidinones
5 via
a 5-exo-tet process. Theoretical calculations confirm the preference
of intramolecular versus intermolecular nucleophilic attack in threo isomers
3a-d.
1. a) Agami, C.; Couty, F.; Hamon, L.; Venier, O. J. Org. Chem. 1997, 62, 2106-2112. b) García-Valverde, M.; Pedrosa, R.; Vicente, M; García-Granda, S.; Gutierrez-Rodriguez, A. Tetrahedron,1996, 52, 10761-10770.
2. Nicolau, K. C.; Sorensen, E. J. Clasics in Total Synthesis; VCH: New-York, 1996
3. Azuma H.; Tamagaki, S.; Ogino, K. J. Org. Chem. 2000, 65, 3538-3541. Moore, J. W. Luzzio, F.A. . TetrahedronLett. 1995, 36, 6599-6602. Bernardi, A.; Cardani, S.; Scolastico, C.; Villa, R. Tetrahedron, 1990, 46, 1987-1998. Cardani, S.; Genari, C.; Scolastico, C.; Villa, R. Tetrahedron, 1989, 45, 7397-7404.
4. Jordá-Gregori, J.M.; González-Rosende, M.E.; Sepúlveda-Arques, J.; Galeazzi, R.; Orena, M. Tetrahedron: Asymmetry 1999,10, 1135-1143. Sepúlveda-Arques, J.; Armero-Alarte, T.; Acero-Alarcón, A.; Zaballos-García, E.; Yruretagoyena, Solesio, B.; Ezquerra-Carrera, J. Tetrahedron, 1996, 52, 2097-2102.
5 Garner, P.; Park, J. M. Org. Synth. Coll. Vol. IX 1998, 300. Dondoni, A.; Perrone, D. Synthesis 1997, 527-529. McKillop, A.; Taylor, R. J. K. Watson, R. J.; Lewis, N. Synthesis1994, 31-33. Garner, P.; Park, J. M. J. Org. Chem. 1987, 52, 2361-2364.
6 Beaulieu, P.L.; Duceppe, J.-S.; Johnson, C. J. Org. Chem. 1991, 56, 4196-4204.
7 Moore, J. W. Luzzio, F.A. Tetrahedron Lett. 1995, 36, 6599-6602. Chiral oxiranyl derivative 3a by means of dimethyl sulfinium methylide epoxidation has been obtained in 55 % yield. Protected as the benzyloxy carbamate led only to 20 % of the desired oxirane.
8 Berkowitz, D. B.; Pedersen, M. L. J. Org. Chem. 1995, 60, 5368-5369. Diastereoselective peracid-mediated epoxidations have been reported. It is worthy of note that the most commly accepted model for hydroxyl- or carbamate –directed epoxidations attributes the threo diastereoselectivity usually observed to A(1,3) interactions present in the erythro transition state.
9 Vanucci, C.; Brusson, X.; Verdel, V.; Zana, F.; Dhimane, H.; Lhommet, G. Tetrahedron Lett. 1995, 36, 2971-2974
10 Urabe, H.; Aoyama, Y.; Sato, F. Tetrahedron 1992, 48, 5639-5646. Farr, R. A.; Holland, A. M.; Huber, E. W.; Peet, N. P. Weintraub, P. M., Tetrahedron 1994, 50, 1033-1040. Romeo, S.; Rich, D.H. Tetrahedron Lett. 1993, 34, 7187-7190.
11 Frisch, M.J.; Schelegel, H.B.; Gill, P.M.W.; Johnson, B.G.; Robb,
M.A.; Cheeseman, J.R.; Keith, T.; Peterson, G.A.; Montgomery, J.A.; Raghavachari,
K.; Al-Laham, M.A.; Zakrzewski, V.G.; Ortiz, J.V.; Foresman, J.B.; Cioloswki,
B.B.; Stefanov, B.B.; Nanayakkara, A.; Challacombe, N.; Peng, C.Y.; Ayala,
P.Y.; Chen, W.; Wong, M.W.; Andrés, J.L.; Replogle, E.S.; Gomperts,
R.; Martín, R.L.; Fox, D.J.; Binkley, J.S.; Defrees, D.J.; Stewart,
J.J.P.; Head-Gordon, M.; González, C.; Pople, J.A. GAUSSIAN 94,
Revisión C.: Gaussian Inc.: Pittsburg, PA, 1995
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0007 as the message subject of your e-mail.