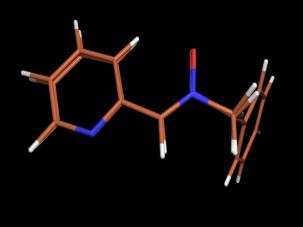
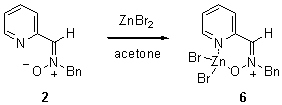[A0008]
Crystal and molecular structures of
N-benzyl-C-(2-pyridyl) nitrone and its ZnBr2 complex.
Pedro Merino*, Sonia Anoro, Tomas Tejero
Laboratory of
Asymmetric Synthesis. Department
of Organic Chemistry. University of Zaragoza. E-50009 Zaragoza. Aragon. Spain
E-mail: [email protected]
Mariano Laguna, Elena Cerrada, Ana Moreno
Department of Inorganic Chemistry. ICMA. University
of Zaragoza. E-50009 Zaragoza. Aragon. Spain
E-mail: [email protected]
Received: 8 July 2000 / Uploaded: 26 July 2000
Synthesis and
Structural Analysis
Introduction
Lewis acid
modulated reactions play a very important role in organic synthesis as they provide
versatile intermediates, sometimes dictating the stereochemistry of the products (1). Nitrones are substrates rather sensitive to Lewis-acid modulation as
we have amply demonstrated during the last years (2) in nucleophilic
additions to chiral nitrones. For instance, it has been demonstrated in our laboratory
that by employing either ZnBr2 or Et2AlCl, one can produce
hydroxylamines with either syn or anti stereochemistry (with respect to the alfa
group), respectively (3).
1,3-Dipolar
cycloaddition reactions with nitrones are also susceptible to be influenced by the
presence of Lewis acids. In fact, Lewis acids catalyzed nitrone cycloadditions have been
extensively studied (4). Kanemasa and Tsuruoka have suggested the
participation of nitrone-MgBr2 complexes in some cycloaddition reactions with
allylic alcohols (5). The scope and limitations of chiral Mg(II) and
Cu(II) complexes on the selectivity of cycloaddition of nitrones with alkenes have been
studied by Jorgensen and coworkers (6). Activation of nitrones by chiral
Lewis acids towards cycloadditions with electron-rich alkenes have also been reported (7).
In the context of cycloaddition
chemistry, we have recently reported the 1,3-dipolar cycloaddition of several hetaryl
nitrones 1-5 with both electron-rich (8) and electron-deficient alkenes (9). The asymmetric
version of the cycloaddition reaction between C-(2-furyl)-N-benzyl nitrone 1 and acrylates has been used in the preparation
of various protected derivatives of 4-hydroxy-pyroglutamic acids of synthetic utility (10).
With the aim of modulating the
reactivity of hetaryl nitrones 1-5 we have started a project directed to understand
the properties of Lewis acid complexes with compounds 1-5. Stable complexes of nitrones have already
been studied. Nitrone complexes of iron have been full-characterized and their acidic
hydrolysis studied by Pierre and coworkers (11). The same authors have
also reported the electrochemical reduction of such complexes (12).
Several tin (IV) complexes with nitrones giving pentacoordinated metal compounds have been
prepared (13). Crist and coworkers (14) have
prepared and characterized complexes of N-tert-butyl-C-(2-pyridyl) nitrone with Cu(II),
Mn(II), Co(II), Ni(II), Fe(II) and Fe(III). The X-ray structures of some complexes have
also been determined (15). Boron chelates with some particular aryl
nitrones have also been described (16).
In this communication we describe the
structural and theoretical studies of both (Z)-N-benzyl-C-(2-pyridyl) nitrone and its ZnBr2
complex. Additionally, we also compare the reactivity of the nitrone alone and the
isolated complex in cycloaddition reactions with vinyl acetate.
Synthesis
and Structural Analysis
The N-benzyl-C-(2-pyridyl) nitrone 2 was prepared by condensation of
pyridine-2-carbaldehyde (17) and N-benzylhydroxylamine (18)
following our previously reported procedure (19). Compound 2 was a crystalline stable product and showed
a Z-configuration as demonstrated by nOe experiments and X-ray crystallography.
Transparent blocks of 2 were grown at room
temperature by slow evaporation of a 1:1 hexane/EtOAc mixture. X-ray
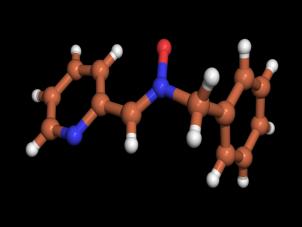
diffraction data were obtained at 173 K and the structure is given in Figure 1.
Selected data are given in Table 1.
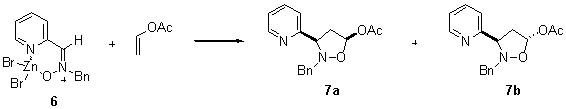
The reaction of 2 with ZnBr2 in acetone for 1 h gave
the crystalline chelate 6 after precipitation
with diethyl ether (Scheme 1) which displayed a signal for the azomethine proton at d 5.40 ppm
in contrast to the signal at d 5.19 ppm displayed by nitrone 2 for the same proton. This result indicated the
different orientation of the nitrone group induced by the complexation with the Lewis
acid.
|
|
Figure 1. X-ray structure of
nitrone 2 |
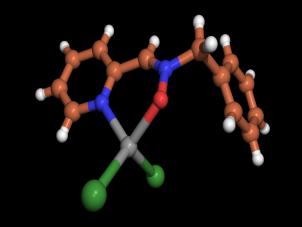
Figure 2. X-ray structure of
complex 6 |
The complex 6 crystallized as transparent blocks suitable for
X-ray analysis from a 1:1 CH2Cl2/hexane mixture. The X-ray diffraction data were obtained at 243 K (due to some
instability of the crystal at lower temperatures) and the structure is given in Figure 2.
This structure provided an opportunity to compare the structure of 6 with 2.
In fact, the features of the solid-state geometry of 6 summarized in Table 1,
are discussed in comparison with the values for 2.
Scheme 1
A coplanarity is seen, as in other
hetaryl nitrones (20), between the planes of the heterocycle moiety and
the nitrone function in both the nitrone alone and the chelate with ZnBr2. As
expected, formation of the complex resulted in some bond length and bond angle differences
between 2 and 6 (Table 1). It is
worthwhile noting the change of the N2-C3-C4-N5 dihedral angle as a consequence of the
formation of the chelate. In the complex the metal atom adopts an almost tetrahedral
disposition the bond angles with bromine atoms being larger than those with nitrogen and
oxygen atoms.
X-ray
diffraction data
Crystallographic data for compounds 2 and 6
appear in Table 1. The final unit-cell parameters were obtained by
least squares on the settings angles for 39 reflections with qmin/max = 9.49-24.75 deg. for 2 and 32 reflections with qmin/max =
10.35-24.56 deg. for 6. Intensity data were
measured on a Siemens P4 diffractometer using the w-2q scan technique. The
structures were solved by direct methods and all non-hydrogen atoms were refined
anisotropically. The hydrogen atoms were located at calculated positions.
Selected acquisition data
|
||
|
2 |
6 |
Formula
|
C13H12N2O |
C13H12Br2N2OZn |
FW |
212.25 |
437.44 |
Crystal system |
orthorhombic |
triclinic |
Space group |
Pbcn |
P-1 |
a (┼) |
11.033 (5) |
7.303 (2) |
b (┼) |
11.107 (5) |
8.135 (3) |
c (┼) |
17.414 (5) |
12.970 (6) |
a (deg.) |
90 |
87.01 (3) |
b (deg.) |
90 |
80.56 (3) |
g (deg.) |
90 |
84.60 (3) |
V (┼3) |
2134.0
(15) |
756.2
(5) |
Z |
8 |
2 |
rcalc (g/cm3) |
1.321 |
1.921 |
F(000) |
896 |
424 |
m (Mo-Ka), cm-1 |
0.086 |
6.900 |
crystal size, mm |
0.38x0.20x0.16 |
0.24x0.10x0.08 |
measurement T (K) |
173 (2) |
243 (2) |
qmax (deg.) |
25.11 |
25.0 |
crystal decay (%) |
6.86 |
15.4 |
total reflections |
2446 |
5169 |
total unique reflections |
1886 |
2628 |
Rmerge |
0.072 |
0.097 |
reflections I>2s(I) |
1083 |
1333 |
No. parameters |
147 |
174 |
R |
0.0474 |
0.1007 |
Rw |
0.0847 |
0.2432 |
GoF (S) |
1.084 |
1.162 |
Siemens P4 diffractometer. Mo-Ka
radiation (l=0.71609┼), normal
focus sealed tube, graphite monochromator. Values given for R, Rw and GoF are based on
total unique reflections. Computing data collections: Siemens
XSCANS (21). Structure solution:
SIR-97 (22). Structure refinement: SHELXL-97 (23). Molecular Graphics: PovChem v 2.1 (24) |
||
Theoretical
Calculations
In order to assess the various factors
contributing to the structural differences between 2
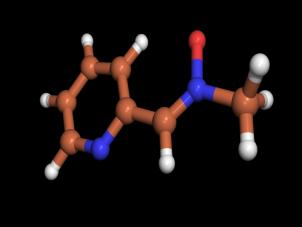
and 6 we have carried out ab initio energy calculations. The optimized structures for 2 and 6
are shown in Figures 3 and 4.
|
|
Figure 3. Optimized structure
(HF/3-21G) for 2 |
Figure 4. Optimized structure
(HF/3-21G) for 6 |
The molecular structures found with ab
initio calculations agree with those observed in the crystalline state although some
differences were observed. For instance, theoretical calculations gave longer O1-N2 bonds
and shorter N2-C3 bonds than those found in crystalline state, presumably due to the
consideration of resonance for the nitrone function. Nevertheless, the modeled structures
showed a good overlap with the X-ray structures (Figures 5 and 6).
|
|
Figure 5. Comparison of the modeled
structure of nitrone 2 with the X-ray
structure. |
Figure 6. Comparison of the modeled
structure of complex 6 with the X-ray structure. |
Ab initio calculations
Calculated structures were optimized at
HF/3-21G level using Gaussian98 (25). All internal coordinates were
free. In both 2 and 6 the benzyl group has been replaced for a methyl
group, and in the case of 6 the Br atoms have
been replaced by Cl atoms. The X-ray data were used (after replacement of the indicated
groups) to generate guessed structures which were pre-optimized at semiempirical level
(PM3) using MOPAC97 as implemented in CS ChemOffice (26). In the case
of the nitrone alone a complete conformational analysis has been carried out in order to
determine the preferred orientation of the nitrone function with respect to the pyridine
ring.
Table 1. Selected bond lengths
(┼), bond angles (░) and dihedral angles (░) for 2 and 6.

numbering scheme
|
Nitrone 2 |
Complex 6 |
||
|
X-ray data |
HF / 3-21G |
X-ray data |
HF / 3-21G |
bond lenghts |
||||
O1-N2 |
1.299 |
1.383 |
1.396 |
1.326 |
N2-C3 |
1.300 |
1.268 |
1.261 |
1.228 |
C3-C4 |
1.451 |
1.458 |
1.469 |
1.501 |
C4-N5 |
1.365 |
1.337 |
1.318 |
1.318 |
N2-C10 |
1.488 |
1.472 |
1.477 |
1.500 |
O1-Zn |
-------- |
-------- |
1.993 |
1.930 |
N5-Zn |
-------- |
-------- |
2.055 |
2.029 |
Zn-Br1 |
-------- |
-------- |
2.321 |
2.287 |
Zn-Br2 |
-------- |
-------- |
2.357 |
2.333 |
bond angles |
||||
O1-N2-C3 |
126.1 |
125.6 |
127.3 |
123.5 |
N2-C3-C4 |
126.3 |
126.4 |
129.8 |
125.7 |
C3-C4-N5 |
113.2 |
113.8 |
118.6 |
119.6 |
O1-N2-C10 |
114.4 |
111.2 |
111.1 |
111.2 |
C3-N2-C10 |
119.5 |
123.2 |
121.0 |
125.3 |
O1-Zn-N5 |
-------- |
-------- |
89.7 |
87.5 |
N2-O1-Zn |
-------- |
-------- |
118.1 |
111.1 |
N5-Zn-Br1 |
-------- |
-------- |
108.1 |
105.3 |
O1-Zn-Br2 |
-------- |
-------- |
113.7 |
126.1 |
dihedral
angles |
||||
O1-N2-C3-C4 |
3.1 |
0.0 |
2.6 |
2.9 |
N2-C3-C4-N5 |
175.6 |
180.0 |
13.0 |
23.7 |
O1-N2-C10-C11 |
112.6 |
-------- |
-------- |
85.0 |
N2-O1-Zn-N5 |
-------- |
-------- |
122.8 |
141.5 |
O1-Zn-N5-C4 |
-------- |
-------- |
37.2 |
30.7 |
Reactivity
The reactivity of the complex was
studied by condensing it with vinyl acetate in a 1,3-dipolar cycloaddition reaction. We
have recently reported (8) the reaction between nitrones 1-5 and
vinyl acetate to give the corresponding isoxazolidines. It might be expected a difference
of reactivity between 2 and 6. However, only slight differences in both
reactivity and selectivity were found. Data for the reactivity are illustrated in Scheme
2. The results of the same reaction with nitrone 2 are also given for comparison.
Scheme 2
1,3-Dipole |
7a : 7b |
yield (%) |
2 |
85 : 15 |
85 |
6 |
89 : 11 |
87 |
Conclusion
In summary, this preliminary results
showed the possibility of using complexed nitrones as suitable 1,3-dipoles in
cycloaddition reactions. Similar structures were found in the crystal structure and by the
ab initio calculations both for nitrone 2 and
ZnBr2-complex 6. The reactivity of
those compounds were compared by condensing them with vinyl acetate. Only slight
differences of reactivity were observed between the nitrone alone and the complex. Further
investigations with other complexes are now in progress.
The authors gratefully acknowledge the
financial support given by the DGES (Project PB97-1014. Madrid. Spain)
References
(1) (a) Yamamoto, Y. Angew. Chem. Int. Ed. Engl. 1986,
25, 947. (b) Narasaka, K. Synthesis, 1991, 1. (c) Suzuki, K. Pure Appl. Chem. 1994, 66,
1557.
(2) For a recent account see: Merino, P.; Franco, S.; Merchan,
F.L.; Tejero, T. Synlett 2000, 442.
(3) See inter alia:
(a) Merino, P.; Castillo, E. Franco, S.; Merchan, F.L.; Tejero, T. J. Org. Chem. 1998,
63, 2371. (b) Merino, P.; Franco, S.; Merchan, F.L.; Tejero, T. Tetrahedron:
Asymmetry 1997, 8, 3489.
(4) Kanemasa, S.; Uemura, T.; Wada, E. Tetrahedron Lett. 1992, 33,
7889. For a
review see: Kanemasa, S.; Oderaotoshi, Y. J. Synth.
Org. Chem. Jpn. 1998, 56, 368.
(5) Kanemasa, S.; Tsururoka, T. Chem. Lett. 1995,
49, 123
(6) (a) Gothelf, K.V.; Hazell, R.G.; Jorgensen, K.A. J. Org. Chem. 1996, 61,
346. (b) Gothelf, K.V.; Hazell, R.G.; Jorgensen, K.A. J. Org. Chem. 1998, 63,
5483. (c) Jensen, K.B.; Gothelf, K.V.; Hazell, R.G.; Jorgensen, K.A. J. Org. Chem. 1997, 62,
2471.
(7) Simonsen, K.B.; Bayon, P.; Hazell, R.G.; Gothelf, K.V.;
Jorgensen, K.A. J. Am. Chem. Soc. 1999, 121, 3845.
(8) Merino, P.; Anoro, S.; Merchan, F.L.; Tejero, T. Molecules,
2000, 5, 132.
(9) (a) Tejero, T.; Dondoni, A.; Rojo, I.; Merchan, F.L.;
Merino, P. Tetrahedron 1997, 53, 3301. (b) Merino, P.; Anoro, S.;
Merchan, F.L.; Tejero, T. Heterocycles, 2000, 53, 861.
(10) Merino, P.; Anoro, S.; Franco, S.; Merchan, F.L.; Tejero,
T.; Tu˝on, V. J. Org. Chem. 2000, 65, 1590.
(11) Pierre, F.; Moinet, C.; Toupet, L. J. Organomet. Chem. 1997, 527,
51.
(12) Pierre, F.; Stricker, A.; Moinet, C.; Sinbandhit, S.;
Toupet, L. J. Organomet. Chem. 1998, 553,
253.
(13) Alallaf, T.A.K.; Abdulrahman, A. Synth. React. Inorg. Metal-Org. C 1997, 27,
985.
(14) (a) Villamena, F.A.; Dickman, M.-H.; Crist, D.R. Inorg. Chem. 1998, 37,
1446. (b) Villamena, F.A.; Crist, D.R. J. Chem. Soc.
Dalton, Trans. 1998, 4055.
(15) Dickmann, M.-H.; Ward, J.P.; Villamena, F.A.; Crist, D.R. Acta Cryst. Sect. C Cryst. Struct. Commun. 1998, 54,
929.
(16) Kliegel, W.; Metge, J.; Rettig, S.J.; Trotter, J. Can. J. Chem. 1998, 76,
1082.
(17) Purchased from Aldrich (P6,200-3) and distilled prior to
use
(18) Borch, R.F.; Berstein, M.D.; Durst, M.D. J. Am. Chem. Soc. 1971,
93, 2897.
(19) Dondoni, A.; Franco, S.; Junquera, F.; Merchan, F.L.;
Merino, P.; Tejero, T. Synth. Commun. 1994, 24, 2537.
(20) Merino, P.; Anoro, S.; Tejero, T.; Laguna, M.; Cerrada,
E.; Moreno, A. unpublished results.
(21) Siemens XSCANS. X-Ray Single Crystal Analysis System.
Copyright (C) 1992 by SIEMENS. Siemens analytical X-ray instruments Inc. Madison,
Wisconsin. USA
(22) SIR-97. A Package for Crystal
Structure Solution by Direct Methods and Refinement. Giacovazzo
et al. 1997
(23) SHELXL-97. Program for the Refinement of Crystal Structures. Sheldrick, G. M. 1997.
(24) PovChem v. 2.1. Copyright (C) 1999 by Paul A. Thiessen.
(25) Gaussian 98, Revision A.3, M. Frisch, J.; Trucks, G. W.;
Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.;
Cheeseman, J. R.; Zakrzewski, V. G.; Montgomery Jr., J. A.; Stratmann, R. E.; Burant, J. C.; Dapprich, S.; Millam, J. M.;
Daniels, A. D.; Kudin, K. N.; Strain, M. C.;
Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.;
Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford,
S.; Ochterski, J.; Petersson, G. A.; Ayala, P. Y.; Cui, Q.; Morokuma, K.; Malick,
D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J.
B.; Cioslowski, J.; Ortiz, J. V.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R.
L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng,
C. Y.; Nanayakkara, A.; Gonzalez, C.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen,
W.; Wong, M. W.; Andres, J. L.; Gonzalez, C.;
Head-Gordon, M.; Replogle, E. S. and Pople, J. A. Gaussian,
Inc., Pittsburgh PA, 1998.
(26) CS ChemOffice. Copyright (C) 1997 by Cambridge Soft
Corporation. Cambridge, MA. USA