|
4th International Electronic Conference on
Synthetic Organic Chemistry (ECSOC-4),
www.mdpi.org/ecsoc/,
September 1-30, 2000
|
|
|
| Contents: |
|
1. Introduction
|
|
Previous work on differently substituted carbostyrils [1-3] has shown that only some especially substituted molecules of this substance class show interesting fluorescence properties. However, in contrast to the widely used oxa analogue coumarins strong fluorophors are scarce. Therefore principle strategies have been investigated, which substituent properties are important. What makes a carbostyril to be a good fluorophor [3]
Experimental and theoretical data have shown that the position 7 influences properties like absorption and quantum yield, whereas position 6 is connected to absorption wavelength. A strong acceptor substituent in position 4 gives a significant bathochromic shift and increases the quantum yield in most of the investigated molecules.  Especially acylation effects on amino functions are very interesting. The only commercially available 4-methyl-7-amino-2(1H)quinolinone (cpd 10 in this paper), sold as "carbostyril 124" or "CS 124" , 4-methyl-7-dimethylamino-2(1H)quinolinone "CS 165" and the esterase substrate 7-hydroxy-4-trifluoromethyl-2(1H)quinolinone "CS151" have very high quantum efficiency (the latter two not in this paper). However, as can be seen in the table, N7-acylation of CS 124 shifts the absorption-and emission-maxima significantly to the blue and quantum yield drops drastically.(cpd 12 in this paper).
Therefore in this work the influence of amino, alkylamino and acetylamino groups in positions 6 or 7 in combination with a methoxy group in position 7 or 6 on absorption and emission properties is investigated. In the context to CS 124, our standard 6,7-dimethoxy-4-trifluoromethyl-2(1H)quinoline 1, which has been published recently [4], will be named CS370. 6-acetylaminocarbostyrils 14 and trifluormethylanalogue 15 were not synthesized, however, they were calculated for comparison.
|
|
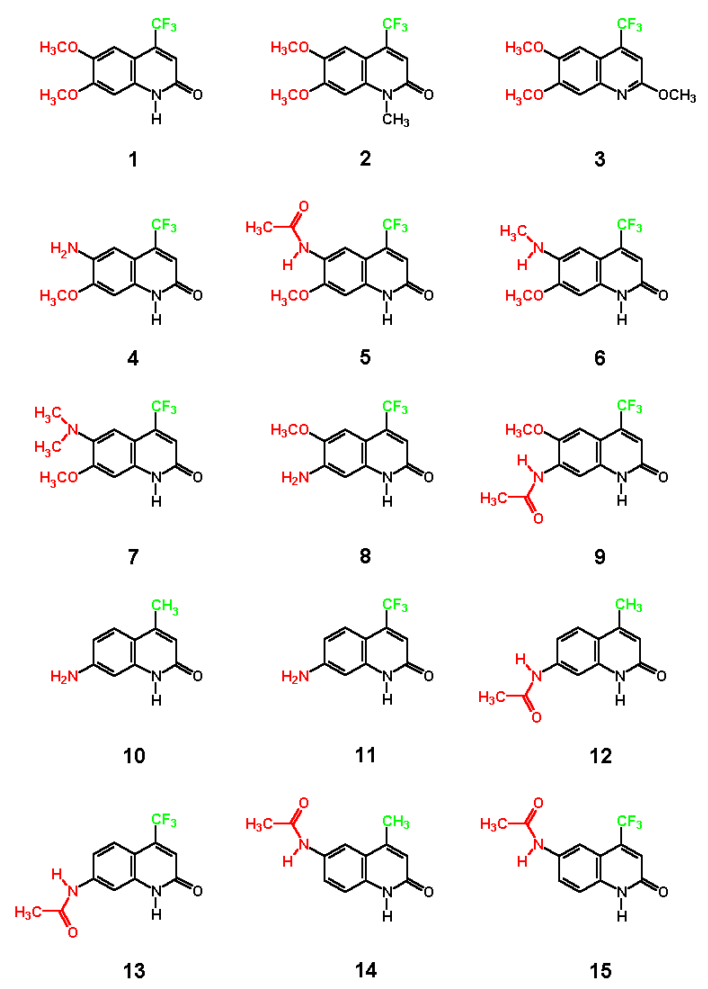
2. Structure of the investigated compounds
|
|
|
|
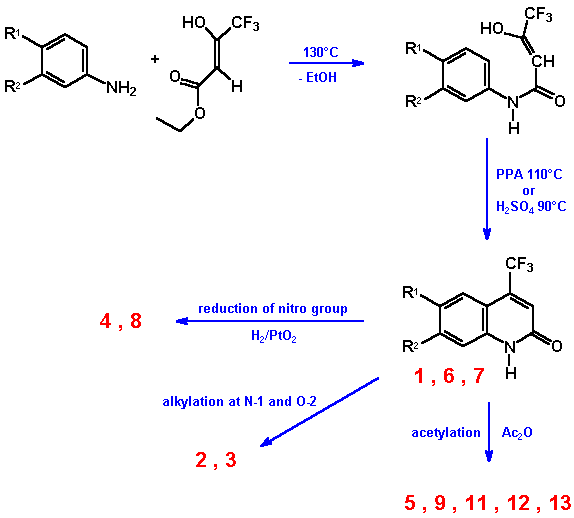
3. Synthesis
|
|
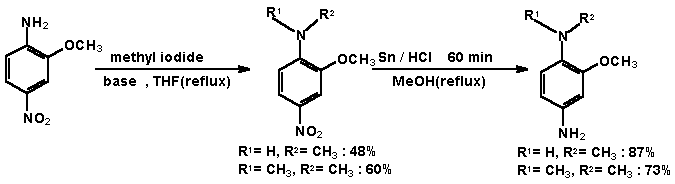
The following anilines have already been described in literature: 2-methoxy-N-methyl-4-nitro-aniline [5], 2-methoxy-N,N-dimethyl-4-nitro-aniline [6] and 2-methoxy-1-N-methyl-4-phenylenediamine [7]. |
|
|
|
4. Spectroscopic Investigation
|
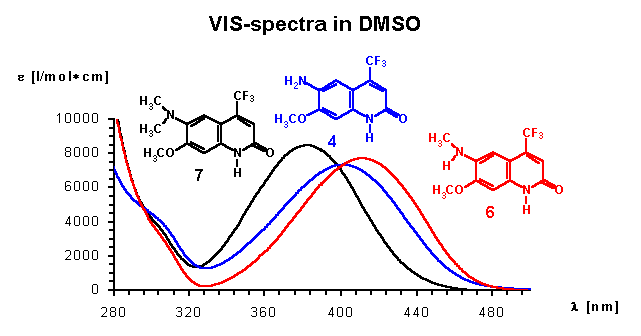
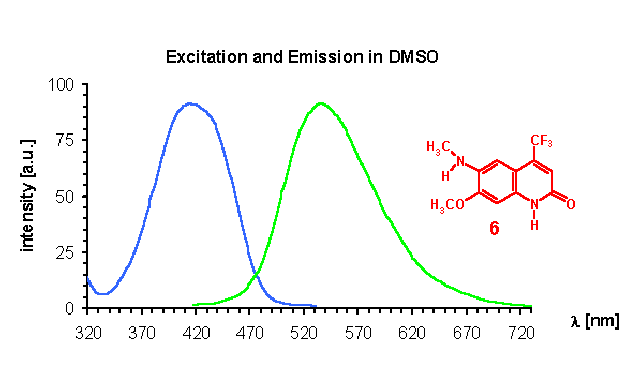
As known for our model 2(1H)-quinolinone CS370 1 , the newly described N-methyl derivative 2 also shows no significant difference in absorption and emission maxima to the parent compound. The observed solvent dependence is consistent with previous observations leading to significant blue shifts (about 10-15 nm in water compared with DMSO). However, O-Methylation and hence formation of a quinoline structure 3 leads to significant shorter wavelength absorption and especially small Stoke´s shifts. This is a very good indicator for a quick analysis if O or N-alkylation has occurred. A comparison of the UV-spectra of 6-amino 4 vs methylamino 6 and dimethylaminoderivative 7 shows surprisingly the dimethyl derivative 7 having the lowest longwave absorption maximum. As expected, N-acetylamino derivative 6 is most blue shifted. This is also reflected in the calculation of the lowest energy structure showing the sterically more demanding dimethylamino group out of plane. However, it is very interesting also from a theoretical point of view, that the Stoke shifts of 173 nm or 214 nm as well as the emission maxima of 557 or 580 nm (in DMSO or water)are the highest values ever observed on a carbostyril. Excitation and emission spectra of this interesting compound are displayed below. Surprisingly, our compounds believed to be of structure 8 and 9 show too low wavelength absorption maxima as evidenced by comparison with analogues 7 and 8 (see yellowish fields in table). The calculated values support strongly, that we should think about revision of these structures, because they could be unexpected isomers. We are continuing to get a confirmation about this subject.
|
|
5. Calculations
|
|
The geometries of all investigated compounds were completely optimized by the semiempirical AM1 Hamiltonian [9] using the eigenvector following routine [10] (keyword PRECISE) as implemented in the Vamp program package [11]. Electronic excitation energies were obtained by the ZINDO program [12,13]. Bulk solvent effects (DMSO, H2O) were included by the self-consistent reaction field (SCRF) approximation [14,15].
|
create permanent table window;
view calculated 3D-structures: click number in table!
compound solvent e l l f l Stokes’ shift DMSO H2O 10670 11560 358 368 366 0.351 0.352 444 432 76 74 0.452 0.241 DMSO H2O 6680 6680 370 360 369 366 0.338 0.339 438 430 68 70 0.344 0.135 DMSO H2O 9290 8070 345 344 344 0.148 0.148 383 383 35 38 0.247 DMSO H2O 7350 8720 379 372 369 0.134 0.315 533 531 130 152 0.160 0.071 DMSO H2O 7330 6590 353 353 352 0.284 0.285 448 440 80 87 0.278 0.161 DMSO H2O 7955 7040 414 389 376 372 0.312 0.313 532 544 118 155 0.274 0.087 DMSO H2O 8470 9000 366 351 350 0.335 0.356 557 580 173 214 0.120 0.070 DMSO H2O 6200 361 362 360 0.372 0.373 406 45 0.208 DMSO H2O 6100 6000 342 340 372 369 0.342 0.343 376 382 34 42 0.124 0.165 DMSO H2O 16670 15800 354 341 337 333 0.505 0.509 388 416 34 75 0.485 0.959 DMSO H2O 374 344 341 0.470 0.473 448 74 DMSO H2O 10400 9540 336 327 322 322 0.210 0.210 371 366 35 39 0.037 0.096 DMSO H2O 346 340 338 0.426 0.430 400 54 DMSO H2O 348 346 0.146 0.146 DMSO H2O 348 347 0.177 0.177
6. Conclusion
As shown in our recent publication, push-pull substituted carbostyrils in position 6,7 and 4, respectively, have absorption maxima close to the visible region.
The large bathochromic shifts are coupled mainly due to the influence of electron donating groups in position 6 of the ring system. Monoalkylated 6-amino groups in combination with a 7-methoxy group give the largest red shifts (UV maximum: 414 nm for compound 6 in DMSO), whereas dialkylated 6-amino groups give the largest Stokes shifts (173 nm in DMSO respectively 214 nm in water for compound 7). It is interesting to note that acetylated 6-amino groups have about the same impact on photophysical properties as methoxy groups (compare 1 with 5). Quantum yields are typically at least 0.1. As expected, non acylated amino substituents in position 7 exhibit the strongest electron donating effects increasing quantum yields significantly. In most of the investigated cases intensities are lower in water as solvent compared with polar-aprotic DMSO. From our previous experience [3,4] one can expect quite good agreement between calculated and experimental absorption maxima. There is, however, one notable exception: the donor properties of amino groups, and hence the bathochromic shift induced thereby, is greatly underestimated by the ZINDO procedure (see e.g., results for 4, 6, 10, 11). This seems to be a quite general shortcoming of this semiempirical quantum chemical method. For the other derivatives the agreement between experiment and calculation is quite satisfying. From the present results one can conclude that an acylamino group is comparable in its effect on absorption maxima to an alkoxy function.
According to this set of data we can suggest that 6,7-diamino substituted carbostyrils, especially the ones with monoalkylated amino groups should provide more excellent photophysical properties compared with the compounds presented in this paper. This new fluorophors can serve as potent competitors to the widely used coumarins especially because of the advantage of a better photochemical and thermal stability.
In short we have prepared highly fluorescent carbostyrils with absorption maxima up to 414 nm and it is evident that this range can still be extended preparing 6,7-bis-N-alkyl derivatives.
7. Experimental
Synthesis of Carbostyrils: General Procedure
Carbostyrils were prepared by Knorr reaction, which afforded anilines and b-keto esters. Substituted anilines were added to hot ethyl (or isopropyl) 4,4,4-trifluoroacetoacetate and heated until the removal of alcohol had stopped. Then cyclisation was performed in concentrated sulfuric acid or polyphosphoric acid. The overall yield was about 30 to 60 % in most of the cases. Carbostyrils that resulted from nitro-anilines were reduced under heterogeneous catalysis (PtO2/H2) to give the desired amino derivative.
Measurement of UV- and Fluorescence Spectra
The measurement of the uv spectra was performed at concentrations of 4×10-5 mol/l. From that data calculation of the absorption coefficient was done according to Lambert-Beer's law:
Fluorescence spectra were recorded at concentrations of 4×10-6 mol/l. The quantum yield was calculated from the area (integration) of the uncorrected emission spectra by comparison with standard substances (quinine sulfate [8], 4-methyl-carbostyril [3]). Calculation was done by Parker's equation.
Instrumentation
The UV/VIS spectra were recorded on a Shimadzu uv/vis scanning spectrophotometer UV-2101PC at room temperature.
Excitation and emission spectra were obtained using a Shimadzu RF-5001 PC spectrofluorophotometer. The spectrofluorophotometer is fitted with a 150W xenon lamp operated as a continuous wave source, slits selectable in 6 steps to produce spectral bandwidths of 1.5, 3, 5, 10, 15 and 20 nm, and an R452-01 photo multiplier. Excitation and emission monochromators: ion-blazed holographic concave grating F/2.5.
8. Acknowledgement
9. References
All comments on this poster should be sent
by e-mail to (mailto:[email protected])
[email protected] with
A0010 as the message subject of your e-mail.
![]()
![]()
![]()
![]()
![]()
![]()
This paper was designed according to a mask created by Prof. Wolfgang STADLBAUER; some measurements and syntheses were performed by Pedro TRAAR and Harald MANG
![]()
![]()