Synthesis of 5-Substituted a,b-Unsaturated g-Lactams from
N-Sulfinyl Azadienes by Iron-mediated Reaction Cascades and Palladium-catalyzed Cyclocarbonylation Strategies
 Patrick Amrhein and Karola Rück-Braun*
Patrick Amrhein and Karola Rück-Braun*
Institut für Organische Chemie, Johannes Gutenberg-Universität,
Duesbergweg 10-14, 55099 Mainz, Germany
E-mail: [email protected]
Received: 1
August 2000 / Uploaded: 2 August 2000
Introduction:
A new synthesis of 5-substituted a,b-unsaturated g-lactams is reported. b-[Cp(CO)2Fe]-Substituted N-sulfonyl1 and N-sulfinyl azadienes 1 react with Grignard reagents or organolithium compounds furnishing 5-substituted a,b-unsaturated g-lactams 3.2,3 These reaction cascades occur by initial attack of the organometallic reagent at the imine functionality followed by an intramolecular cyclocarbonylation step. In a number of cases non-N-protected lactams are obtained from N-sulfonyl azadienes besides the desired N-sulfonyl g -lactams 3. Moreover, chiral b-[Cp(CO)2Fe]-substituted N-sulfinyl azadienes 1 react with the organometallic reagents to furnish exclusively 5-substituted non-N-protected g -lactams 3.2,3
In addition, the synthesis of 5-substituted a,b-unsaturated g-lactams 3 by palladium-catalyzed cyclocarbonylations of chiral bromo-substituted sulfinamides 2, derived from the corresponding azadiene precursors and organometallic reagents, is described.4
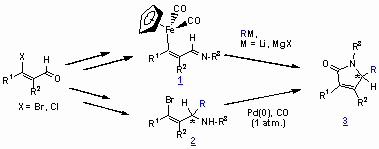
Reactions of b-[Cp(CO)2Fe]-substituted N-Sulfonyl Azadienes with C-Nucleophiles:
a,b-Unsaturated imines 1 are synthesized e.g. from b-[Cp(CO)2Fe]-substituted (Z)-alkenals 4 and electron poor primary amino compounds in CH2Cl2 in the presence of TiCl4 and NEt3 in dichloromethane (66-100 %).1,4

The g-lactams 3 are accessible by treatment of the a,b-unsaturated imines 1 with organolithiums in THF at -78°C or 0°C followed by prolonged stirring at room temperature (39-56 %). Reactions with Grignard-reagents carried out in dichloromethane at room temperature furnished the g-lactams 3 in 37-62 % yield.
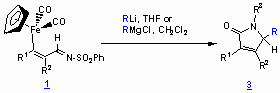
Examples:
The reaction of imine 5 with Grignard reagents is found to furnish the allyliron complexes 6. The structure of the allyl-substituted product was elucidated by X-ray analysis. Therefrom an anti-orientation of the metal fragment and the allyl residue at carbon-5 is concluded.

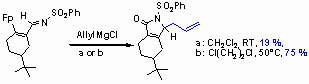
Influence of the temperature on the carbonylation step:
When the cyclohexene-derivative shown below is treated with allyl Grignard reagent at room temperature the N-sulfonyl g -lactam is isolated in only 19% yield. However, the yield could be increased by raising the reaction temperature after the initial 1,2-addition for the cyclocarbonylation key step. Thereby, in 1,2-dichloroethane at 50°C complete turnover to the desired product is achieved. The N-sulfonyl g -lactam is isolated in 75 % yield after flash chromatography.
Unexpected Deprotection: What role does the iron play in this game?
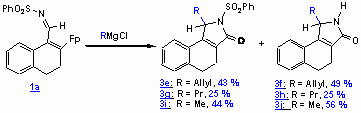
Reactions of compound 1a with either organolithium or Grignard reagents led to the non-N-protected lactams 3f, 3h, 3j besides the lactams 3e, 3g, 3i. At the current stage the cleavage of the N-S bon is not conclusive. Iron-mediated redox processes my be involved in the formation of the non-N-protected g -lactams 3f, 3h, 3j.
Mechanistic Considerations:
After 1,2-additon of the organometallic reagents to the N-sulfonyl azadienes, furnishing the metallated amides (B), the carbonylation steps may proceed via an acyl iron intermediate or a ferrilactam intermediate.1-4
For the formation of the allyliron complexes presented above it seems reasonable to propose an anionic p -alkene-iron complex as intermediate (C) being formed after the reductive elimination step leading to the five-membered ring system. In an intramolecular nucleophilic substitution reaction the attack of the iron moiety at the neighboring methylene group could lead to a phenolate prior aqueous work-up yielding the allyliron complexes.

Application of Iron-substituted N-Sulfinyl Azadienes:
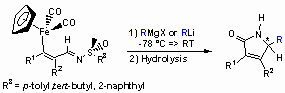
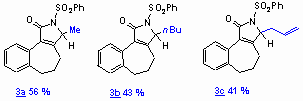
Chiral sulfinimines react with Grignard- or organolithium reagents to give exclusively non-N-protected a,b-unsaturated g-lactams. Only the N-tert-butyl sulfinyl imines can be treated with organolithiums and alkyl Grignard reagents since the sulfur atom is effectively shielded by the tert-butyl residue leading to exclusive attack at the imine carbon atom. For the reactions summarized in the Table, the enantioselectivities were determined by shift experiments to range from 10% ee up to 62% ee.
Iron-mediated redox processes during aqueous work-up seem to be responsible for the N-S bond cleavages observed.
R3 |
Lactam |
R1-M |
Yield |
ee (%) |
|
1 |
p-tolyl |
8 |
AllylMgCl |
64 % |
40 |
2 |
p-tolyl |
8 |
BnMgCl |
27 % |
62 |
3 |
2-naphthyl |
8 |
AllylMgCl |
63 % |
40 |
4 |
tert-butyl |
8 |
AllylMgCl |
45 % |
46 |
5 |
p-tolyl |
7 |
AllylMgCl |
66 % |
52 |
6 |
p-tolyl |
7 |
AllylMgBr |
27 % |
45 |
7 |
tert-butyl |
7 |
AllylMgCl |
55 % |
38 |
8 |
tert-butyl |
7 |
MeLi |
91 % |
10 |
For the addition of organolithiums and Grignard reagents to sulfinimines six-membered ring transition state models have been proposed by Davis and Ellman to predict the diastereofacial selectivity. 5,6

Palladium-catalyzed Cyclocarbonylations:
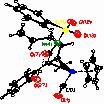
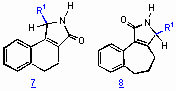
Enantiopure g-lactams 3 are accessible by palladium(0)-catalyzed cyclocarbonylation reactions of chiral sulfinamides. The chiral sulfinyl amides used are synthesized from the corresponding imines by 1,2-addition of organolithium or Grignard-reagents in 50-100 % yield. The diastereoselectivity obtained ranged from 51:49 up to 91:9. Fortunately, the diastereomers are easily separated by chromatography. The absolute configuration of an allyl-substituted and a methyl-substitued optically pure sulfinamide was elucidated by X-ray analysis. In the palladium-catalyzed cyclocarbonylation reactions surprisingly N-sulfinyl-substituted (B) and non-N-protected derivatives (A) are formed. The reactions are carried out with Pd(PPh3)4 and n-Bu3N as base in acetonitrile at reflux for 5-28 h. The N-S bond cleavage can be attributed to attack of hydrogen bromide formed from the palladium hydride intermediate within the catalytic cycle.
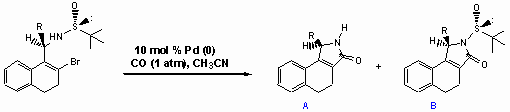
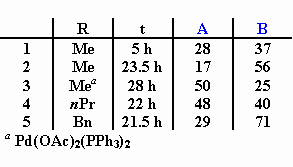
The palladium-catalyzed cyclocarbonylation of allyl-substituted sulfinamides gave the desired lactam derivatives in less than 5% yield. The chiral amines shown below are found to be formed instead by Heck-reaction. In the presence of LiCl compound A is exclusively obtained.
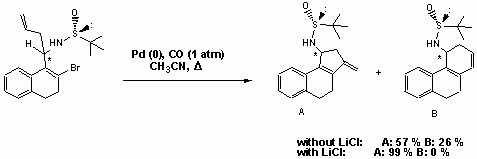
From the optically pure sulfinamide shown below the amine derivative is prepared by treatment with HCl in dioxane/methanol for cyclocarbonylation studies, yielding the non-N-protected a , b -unsaturated g -lactam quantitatively.

In summary, 5-substituted a , b -unsaturated g -lactams were obtained in novel reaction cascades starting from iron-substituted N-sulfonyl or N-sulfinyl azadienes and organometallic reagents. Enantiomerically pure derivatives were prepared from chiral sulfinamides by a palladium-catalyzed cyclocarbonylation strategy starting from b -bromo-substituted N-sulfinyl azadienes.
References:
- K. Rück-Braun, Angew. Chem. Int. Ed. Engl. 1997, 36, 509.
- P. Amrhein, D. Schollmeyer, K. Rück-Braun Organometallics 2000, in press.
- K. Rück-Braun , P. Amrhein Eur. J. Org. Chem. 2000, in press.
- P. Amrhein, D. Schollmeyer, K. Rück-Braun Org. Lett. 2000, submitted for publication.
- F. A. Davis, W. McCoull J. Org. Chem. 1999, 64, 3396.
- D. A. Cogan, G. Liu, J. A. Ellman Tetrahedron 1999, 55, 8883.