
Fig. 1. A novel chiral dinucleating system derived from
tartaric acid esters
Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0016]
Asymmetric 1,3-Dipolar Cycloaddition Utilizing Tartaric Acid Ester as a Chiral Auxiliary
Yutaka Ukaji and Katsuhiko Inomata*
Department of Chemical Science, Graduate School of Natural Science and Technology,
Kanazawa University,
Kakuma, Kanazawa, Ishikawa 920-1192, Japan
Tel: +81-76-264-5700 Fax: +81-76-264-5742 or +81-76-234-4140
E-mail: [email protected]
Received: 8 July 2000 / Uploaded: 29 July 2000
In order to develop a practical method for the construction of chiral molecules, we have designed a novel chiral system possessing two metal centers utilizing tartaric acid esters. Based on this concept, catalytic asymmetric 1,3-dipolar cycloaddition of nitrile oxides and nitrones could be achieved with an high level of stereocontrol.
INTRODUCTION
The development of a practical and efficient method for the
construction of chiral molecules is essential to explore new medicines
and agricultural chemicals. It is obvious that the design of a
specific chiral environment utilizing chiral auxiliaries, both
enantiomers of which are easily available, provides a useful way
to prepare optically active substances. Among such chiral auxiliaries,
tartaric acid esters are one of the most promising and readily
available candidates.
We have designed a novel chiral system possessing two metal centers
utilizing tartaric acid esters. If two reactants were bound to
two different metal centers of the dialkoxide derived from tartaric
acid ester, which might form a rigid 5/5-fused bicyclic dinucleating
structure, they might be ideally oriented and/or activated by
the metals, and the subsequent reaction might proceed in an enantioselective
manner to afford the corresponding optically active products (Fig.
1). According to this hypothesis, we already developed an asymmetric
Simmons-Smith reaction.1)
In this article, we wish to describe asymmetric 1,3-dipolar cycloadditions
of nitrile oxides and nitrones.

Fig. 1. A novel chiral dinucleating system derived from
tartaric acid esters
1. Stoichiometric Asymmetric 1,3-Dipolar Cycloaddition of Nitrile Oxides
Our new idea was as follows: when allyl alcohol (1) was successively treated with diethylzinc, (R,R)-tartaric acid ester, a second diethylzinc, and hydroximoyl chloride, the dinucleating intermediate 2 might be formed by action of ethylzinc as a base toward hydroximoyl chloride to generate the nitrile oxide in situ, and the subsequent 1,3-dipolar cycloaddition was anticipated to proceed in a stereoselective manner to give the corresponding 2-isoxazoline 3 in an optically active form (Eq. (1)).

In accordance with this hypothesis, the asymmetric 1,3-dipolar cycloaddition was performed (Eq. (2)). After optimizing the molar amounts of diethylzinc and hydroximoyl chloride, the corresponding 2-isoxazolines 3 could be obtained in excellent optical yields up to 98% ee, not only for aromatic nitrile oxides but also for aliphatic ones.2) Optimal molar amounts might be related to the reactivity of nitrile oxides toward dimerization and/or the ethylzinc reagent as well as toward allyl alcohol (1) through 2.

Table I. Asymmetric 1,3-dipolar cycloaddition of nitrile oxides to 1
|
Entry |
R |
n |
3 |
Yield/% |
ee/% |
|
1 |
C6H5 |
2.0 |
a |
78 |
96 |
|
2 |
4-CH3OC6H4 |
1.1 |
b |
83 |
98 |
|
3 |
4-ClC6H4 |
1.1 |
c |
74 |
93 |
|
4 |
(CH3)3C |
1.5 |
d |
92 |
96 |
|
5 |
CH3(CH2)5CH2 |
1.5 |
e |
64 |
95 |
2. Catalytic Asymmetric 1,3-Dipolar Cycloaddition of Nitrile Oxides
Among [4p+2p] cycloadditions, many catalytic asymmetric Diels-Alder
reactions using chiral Lewis acids have been reported. By contrast,
the catalytic asymmetric 1,3-dipolar cycloaddition has not yet
met with success. The achievement of a catalytic 1,3-dipolar cycloaddition
of a nitrile oxide was rather difficult because the reagent is
usually too unstable to be isolated and must be generated in
situ.
The 1,3-dipolar cycloaddition of nitrile oxide using a catalytic
amount (0.2 molar amount) of (R,R)-DIPT was examined with
special attention directed to the order of addition and the molar
ratio of the reagents. In the attempts to realize reproducibly
high enantioselectivity, the addition of a small amount of an
ethereal compound such as 1,4-dioxane was found to be markedly
effective. As shown in Table II, the corresponding 2-isoxazolines
3 could be reproducibly obtained with high enantioselectivity
not only for aromatic nitrile oxides but also for aliphatic ones.3)
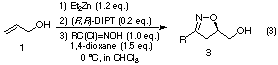
Table II. Catalytic asymmetric 1,3-dipolar cycloaddition of nitrile oxides
|
Entry |
R |
3 |
Yield/% |
ee/% |
||
|
1 |
C6H5 |
a |
87 |
84 |
||
|
2 |
4-CH3OC6H4 |
b |
98 |
90 |
||
|
3 |
4-ClC6H4 |
c |
91 |
90 |
||
|
4 |
(CH3)3C |
d |
91 |
93 |
||
|
5 |
CH3(CH2)5CH2 |
e |
62 |
92 |
||
3. Catalytic Asymmetric 1,3-Dipolar Cycloaddition of Nitrone
The catalytic asymmetric 1,3-dipolar cycloaddition of nitrone 4 to an achiral allyl alcohol (1) also proceeded enantioselectively when a catalytic amount of (R,R)-DIPT was used as a chiral auxiliary to afford cis-isoxazolidine 5 in good optical yield. The addition of an amine oxide such as pyridine N-oxide was essential to realize reproducible stereoselection.4)
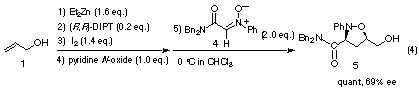
4. Enantioselective Synthesis of (-)-Lasubine II
The utility of this asymmetric 1,3-dipolar cycloaddition in
the challenging arena of total synthesis of natural products was
next explored. The lythraceae alkaloid lasubine II was isolated
from the leaves of Lagerstroemia subcostata Koehne. In
our synthetic strategy for this target molecule, optically active
2-isoxazoline was firstly prepared and then transformed to bicyclic
lasubine II stereoselectively by sequential reduction and cyclization.
The catalytic asymmetric 1,3-dipolar cycloaddition of 3,4-dimethoxybenzonitrile
oxide to allyl alcohol (1) gave the corresponding 2-isoxazoline
5f with excellent enantioselectivity (Eq. (5)).
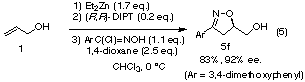
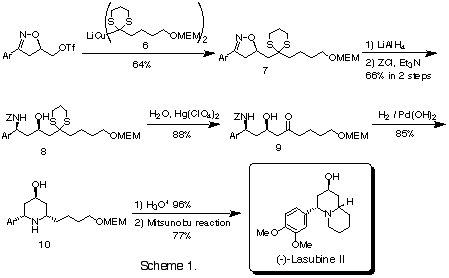
The optically pure 2-isoxazoline 5f obtained by recrystallization was converted to its triflate and coupled with cuprate 6 to give 7. At this stage, the whole carbon skeleton required for the synthesis of lasubine II was arranged. The reduction of isoxazoline 7 followed by the protection of the resulting amino group gave mainly the N-protected syn-amino alcohol 8 along with its anti-isomer. After the hydrolysis of the dithioacetal group, the reductive deprotection of the amino group in b-hydroxy ketone 9 afforded the cis-2,6-disubstituted piperidin-4-ol 10 stereoselectively via spontaneous formation of cyclic imine and subsequent reduction. The amino alcohol derived from 10 by the hydrolysis of the MEM group was cyclized by applying the Mitsunobu reaction to afford optically active (-)-lasubine II.5)
CONCLUSIONS
We have designed a novel chiral system possessing two metal
centers utilizing tartaric acid esters, and asymmetric cycloadditions
have been developed. Although the evidence of a dinucleating species
from X-ray analysisis was not yet shown directly, this concept
can be extended to other asymmetric reactions such as asymmetric
nucleophilic addition,6)
since numerous combinations of metals are possible. Furthermore,
the ready availability of (R,R)- and (S,S)-tartaric
acid esters has made possible the preparation of both enantiomers
of the required substances by simple procedures.
REFERENCES
1. Y. Ukaji, K. Sada, and K. Inomata, Chem. Lett., 1993,
1227.
2. Y. Ukaji, K. Sada, and K. Inomata, Chem. Lett., 1993,
1847.
3. M. Shimizu, Y. Ukaji and K. Inomata, Chem. Lett., 1996,
455.
4. D. Xia, K. Taniguchi, Y. Ukaji, and K. Inomata, 78th National
Meeting of the Chemical Society of Japan, Funabashi, March 2000,
Abstr. 1F138.
5. Y. Ukaji, M. Ima, T. Yamada, and K. Inomata, Heterocycles,
52, 563 (2000).
6. Y. Ukaji, Y. Shimizu, Y. Kenmoku, A. Ahmed, and K. Inomata,
Chem. Lett., 1997, 59-60; Y. Ukaji, Y. Shimizu,
Y. Kenmoku, A. Ahmed, and K. Inomata, Bull. Chem. Soc. Jpn.,
73, 447 (2000); Y. Ukaji, Y, Yoshida, and K. Inomata, Tetrahedron:
Asymmetry, 11, 733 (2000).
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0016 as the message subject of your e-mail.