
[A0022]
Asymmetric Synthesis of Functionalized Prolines by Diastereoselective 1,3-Dipolar Cycloaddition Using Chiral Oxazinone Derivatives
Rafael Chinchilla, Nuria Galindo, Patricia Mazon and Carmen Najera*
Departamento de Quimica Organica, Universidad de Alicante. E-03080 Alicante, Spain. Tel. +34 6 5903728, Fax +34 6 5903549, E-mail: [email protected]
Received: 23 July 2000 / Uploaded: 30 July 2000
Abstract: The reaction of (5S,6S)-6-isopropyl-5-phenyl-1,4-oxazin-2-ones from glycine and alanine with formaldehyde in the presence of electron-deficient olefins afforded the corresponding adducts obtained by a diastereoselective 1,3-dipolar cycloaddition of the chiral azomethine ylids. Hydrolysis of these adducts allows the synthesis of enantiomerically enriched functionalized prolines.
Keywords: Amino acids, oxazinones, 1,3-dipolar cycloadditions, prolines
Introduction
The a -amino acids derived from proline, for example the kainic acid derivatives, present an array of interesting biological properties [1]. The most direct strategy for the synthesis of these functionalized prolines is the 1,3-dipolar cycloaddition reaction of azomethine ylids with electron-deficient olefins [2]. The asymmetric synthesis of these amino acids has been developed using unprotected saturated oxazinones, which have been employed as precursors of the corresponding chiral azomethine ylid dipoles upon reaction with aldehydes, mainly formaldehyde [2].
Recently, we have prepared chiral 3,6-dihydro-2H-1,4-oxazin-2-ones derived from alanine and glycine, which have been used as templates for the asymmetric synthesis of different a -amino acids [3,4]. We report now the use of these heterocycles for the preparation of chiral saturated oxazinones and their use in diastereoselective 1,3-dipolar cycloaddition reactions for the asymmetric synthesis of functionalized prolines.

Results and Discussion
Oxazinones 1 and 2 were prepared as reported [3,4] and hydrogenated [4b,5] to afford the corresponding saturated oxazinones 3 and 4 in 99 and 70% yield, respectively (Scheme 1).Scheme 1

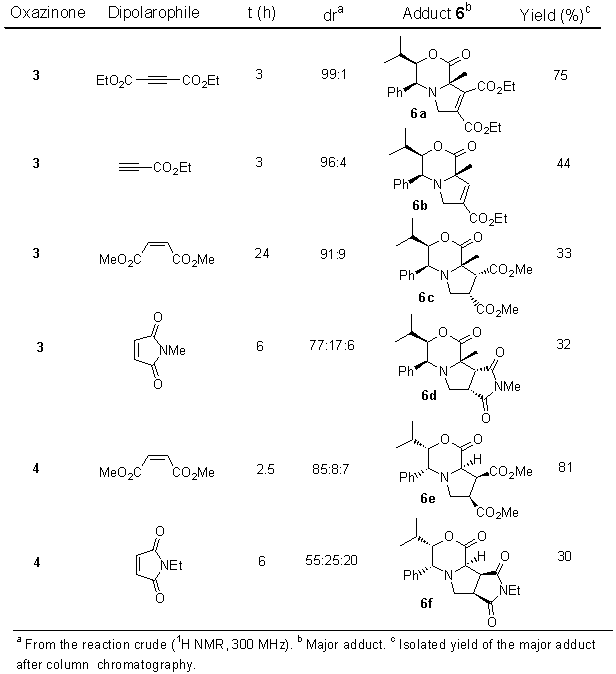
Reaction of oxazinones 3 and 4 with mono- and di-substituted olefins or acetylenes with electron-withdrawing groups in the presence of paraformaldehyde afforded the corresponding adducts 6 from a 1,3-dipolar cycloaddition reaction through the intermediate azomethine ylid 5 (see Scheme 2 and Table 1). The pure major adducts were isolated by column chromatography and their configuration determined by NOE experiments, resulting products of an endo approach. The reaction took place with good diastereoselectivity relative to the phenyl and isopropyl groups.
Scheme 2

Table 1
Hydrolysis of adducts 6 allowed the synthesis of functionalized prolines. For example, adduct 6d was transesterified with HCl (g)/MeOH and hydrogenated at 3.5 bar in the presence of Pd(OH)2 on carbon and TFA [6]. Subsequent hydrolysis with refluxing 6M HCl and treatment with propylene oxide afforded proline derivative 7 (Scheme 3).
Scheme 3
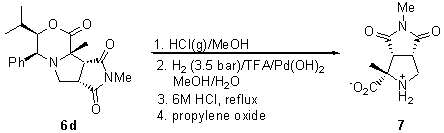
Conclusions
We have prepared chiral saturated oxazinones from alanine and glycine which react with electron-deficient olefins and acetylenes in the presence of paraformaldehyde to give adducts from a diastereoselective 1,3-dipolar cycloaddition reaction. These adducts can be employed for the preparation of functionalized prolines.
Experimental part
General procedure for the cycloaddition reaction: A suspension of the corresponding dipolarophile (5 mmol) and paraformaldehyde (10 mmol, 300 mg) in toluene (25 mL) was heated at 80?C and a solution of the oxazinone 3 or 4 (1 mmol) in toluene (10 mL) was added dropwise. The reaction mixture was stirred at 80?C until completion (GC), cooled at room temperature and filtered through a plug of silica gel (AcOEt). The solvents were evaporated (15 Torr) and the residue was purified by column chromatography (hexane/AcOEt gradients).
Physical and spectroscopical data of adduct 6d follows:
TLC (Hexane/EtOAc 1:1): Rf 0.37.
[a ]D25 +44.0 (c, 1; CH2Cl2).
M. P. 210-211 ?C.
IR (KBr): 1732 and 1704.
1
H-NMR (300 MHz, CDCl3): 0.76 (d, J=6.8Hz, 3H), 1.09 (d, J=6.8Hz,3H), 1.58 (s, 3H), 1.74 (m, 1H), 2.99 (s, 3H), 3.31 (m, 2H), 3.52 (m, 2H), 3.89 (d, J=4.2Hz, 1H), 4.16 (t, J=4.2, 1H), 7.21 (m, 2H) and 7.35 (m, 3H).13
C-NMR (75 MHz, CDCl3): 17.84, 21.11, 25.15, 25.46, 28.30, 43.45, 53.35, 56.27, 63.22, 68.79, 86.71, 128.11, 128.45, 128.97, 136.29, 169.12, 175.84 and 178.30.References
1. See for example: DeShong, P.; Kell, D. A. A total synthesis of (� )-allo-kainic acid. Tetrahedron Lett. 1986, 27, 3979-3982.
2. See for example: Gothelf, K. V.; Jorgensen, K. A. Asymmetric 1,3-dipolar cycloaddition reactions. Chem. Rev. 1998, 98, 863-909. (b) Abellan, T.; Chinchilla, R.; Galindo, N.; Guillena, G.; Najera, C.; Sansano, J. M. Chiral oxazinones and pyrazinones as a -amino acid templates, In Targets in Heterocyclic Systems, Vol. 4, in press.
3. (a) Chinchilla, R.; Falvello, L. R.; Galindo, N.; Najera, C. Asymmetric synthesis of a -methyl a -amino acids by diastereoselective alkylation of optically active 6-isopropyl-3-methyl-2,3-dihydro-6H-1,4-oxazin-2-ones. Angew. Chem. Int. Ed. Engl. 1997, 36, 995-997. (b) Chinchilla, R.; Galindo, N.; Najera, C. Chiral 3,6-dihydro-2H-1,4-oxazin-2-ones as alanine equivalents for the asymmetric synthesis of a -methyl-a -amino acids (AMAAs) under mild reaction conditions. Synthesis, 1999, 704-717.
4. (a) Chinchilla, R.; Falvello, L. R.; Galindo, N.; Najera, C. Asymmetric synthesis of substituted 1-aminocyclopropane-1-carboxylic acids from a new chiral glycine equivalent with 3,6-dihydro-2H-1,4-oxazin-2-one structure. Tetrahedron:Asymmetry 1998, 9, 2223-2227. (b) Chinchilla, R.; Falvello, L. R.; Galindo, N.; Najera, C. New chiral didehydroamino acid derivatives from a cyclic glicyne template with 3,6-dihydro-2H-1,4-oxazin-2-one structure: applications to the asymmetric synthesis of non proteinogenic a -amino acids. J. Org. Chem., 2000, 65, 3034-3041.
5. Caplar, V.; Lisini, A.; Kajfez, F.; Kolbah, D.; Sunjic, V. Synthesis of the 2,3-dihydro-6H-1,4-oxazin-2-ones chiral at C(3) and asymmetric induction in hydrogenation of the azomethine bond. J. Org. Chem. 1978, 43, 1355-1360.
6. Auslow, A. S.; Harwood, L. M.; Lilley, I. A. Synthesis of a -phenyl proline derivatives via 1,3-dipolar cycloaddition of chiral stabilised azomethine ylids. Tetrahedron:Asymmetry 1995, 6, 2465-2468
Acknowledgments: This work was supported by the spanish Ministry of Education and Culture (MEC) (Project no. PB97-0123). N. G. also thanks MEC for a fellowship.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0022 as the message subject of your e-mail.