
 Enzyme-mediated synthesis of (1S)-1-amino-2,2-dimethylcyclopropane-1-carboxylic
acid
Enzyme-mediated synthesis of (1S)-1-amino-2,2-dimethylcyclopropane-1-carboxylic
acidFourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0024]

 Enzyme-mediated synthesis of (1S)-1-amino-2,2-dimethylcyclopropane-1-carboxylic
acid
Enzyme-mediated synthesis of (1S)-1-amino-2,2-dimethylcyclopropane-1-carboxylic
acid
Antonio Salgadoa, Annelies Eeckhauta, Norbert De Kimpea,*
and Johan Van der Eyckenb
aDepartment of Organic Chemistry, Faculty of Agricultural and Applied
Biological Sciences, Ghent University, Coupure links 653, B-9000 Ghent, Belgium;
E-mail: [email protected]
bLaboratory for Organic and Bio-organic Synthesis, Faculty of Sciences, Ghent
University, Krijgslaan 281, B-9000 Ghent, Belgium.
Received: 25 July 2000 / Uploaded: 30 July 2000
Abstract
Hydrolysis of bis(2,2,2-trifluoroethyl) 2,2-dimethylcyclopropane-1,1-dicarboxylate with pig liver esterase afforded (1R)-2,2-dimethyl-1-(2,2,2-trifluoroethoxycarbonyl)-cyclopropane-1-carboxylic acid in high enantiomeric excess. Via a Curtius type reaction with DPPA this compound was rearranged to (1S)-2,2,2-trifluoroethyl-2,2-dimethyl-1-[(N-ethoxycarbonyl)amino]-cyclopropane-1-carboxylate. Final alkaline hydrolysis gave (1S)-1-amino-2,2-dimethylcyclopropane-1-carboxylic acid.
Introduction
a,a-Disubstituted amino acids play an important role in the design of peptides with enhanced properties like resistance to hydrolysis and enzyme cleavage processes [1]. This may be due to the conformational restrictions induced by the incorporation of these residues into peptides. These conformational restrictions are increased when the amino acid moiety is incoporated within a ring structure. Among the 1-aminocycloalkanecarboxylic acids, cyclopropane containing amino acids have attracted the particular attention of many researchers, undoubtedly because they are found to inhibit some amino acid processing enzymes [2].
The smallest member of these cyclopropane containing amino acids, 1-aminocyclopropane-1-carboxylic acid 1 is known to be the biochemical precursor of the plant hormone ethylene in a process catalysed by the ethylene forming enzyme (EFE) [3]. Ethylene, once liberated, induces acceleration of most of the plant development processes, such as flowering, ripening, germination and senescence [4]. Substances that inhibit the ethylene forming enzyme would allow an effective control on the growth of plants, thus being of greatest importance to agriculture [5].
1-Amino-2-substituted-cyclopropane-1-carboxylic acids 2 have received growing interest due to their proven physiological effect as inhibitors of the ethylene forming enzyme [6], thus justifying their role as plant growth regulators. However, little attention has been paid to the geminally substituted 1-amino-2,2-dialkylcyclopropane-1-carboxylic acids 3.
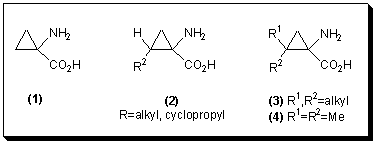
Most of the described syntheses of 1-amino-2,2-dialkylcyclopropane-1-carboxylic acids 3, in particular 1-amino-2,2-dimethylcyclopropane-1-carboxylic acid 4, are limited to the preparation of the racemate [7]. Only a few syntheses of optically active 1-amino-2,2-dimethylcyclopropane-1-carboxylic acid (4) have been reported [8][9][10][11].
All the reported syntheses rely on the use of chiral precursors and auxiliaries, and in the resolution of racemic mixtures. Here, we report the first enantioselective synthesis of (1S)-1-amino-2,2-dimethylcyclopropane-1-carboxylic acid (4) by an enzymatic asymmetrization of a prochiral precursor.
Synthesis of optically active 1-amino-2,2-dimethylcyclopropane-1-carboxylic acid
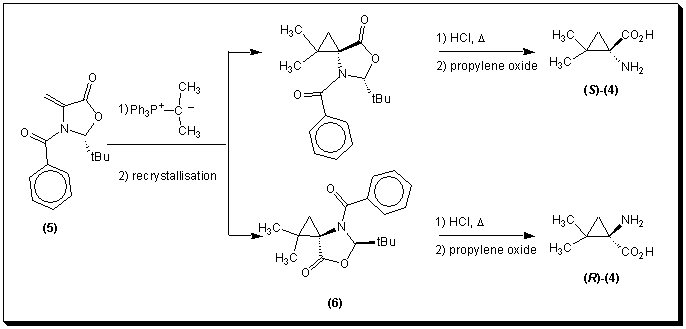
Chincilla et al. [8] described the cyclopropanation of (2S)-N-benzoyl-2-tert-butyl-4-methylene-1,3-oxazolidin-5-one 5 with isopropylidenetriphenylphosphorane at room temperature. Separation of the two spirocyclopropane diastereoisomers 6 by flash chromatography, followed by acid hydrolysis gave the enantiomerically pure (R)- and (S)-2,3-methanovaline 4.
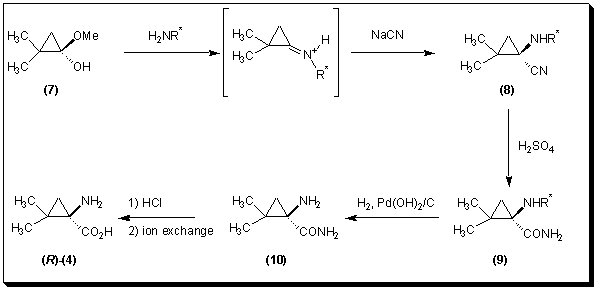
Fadel et al. [9] reported a Strecker-type synthesis. The reaction involved addition of cyanide to a dimethylcyclopropanone hemiacetal 7 in the presence of a chiral amine to provide the corresponding amino nitrile 8 (de 90% before flash chromatography). Hydrolysis of the amino nitrile 8 led to the corresponding amide 9, which upon hydrogenolysis gave amino amide 10. From this amino amide 10 the free amino acid 4 was obtained after acid hydrolysis, followed by ion exchange chromatography.
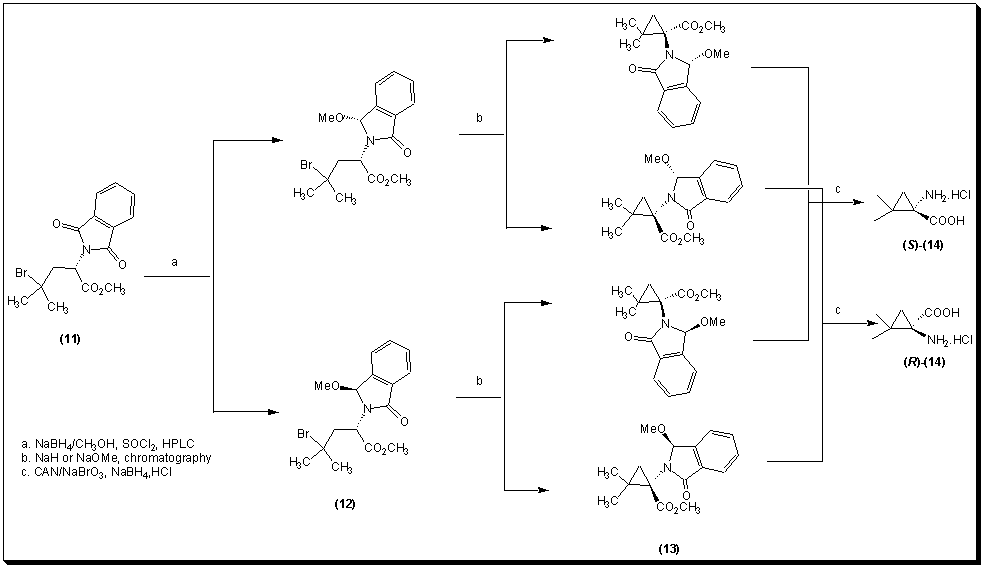
Easton et al. [10] have recently reported the conversion of (S)-leucine into the phthalimide 11. Treatment of the phthalimide with sodium borohydride in methanol gave a pair of diasteriomeric a-methoxyamides 12 that could be separated by HPLC. 1,3-Dehydrobromination of 12 gave the 2,3-methanovaline derivatives 13. Removal of the phthaloyl substituent by successive treatment with ceric ammonium nitrate/sodium bromate and sodium borohydride, followed by acid hydrolysis, released the corresponding amino acid 14 as the hydrochloride.
Kirihata et al. [11] described the fractional crystallisation of the diastereoisomeric salts of 2,2-dimethyl-1-formylaminocyclopropane-1-carboxylic acid with (-)-quinine.
Enzyme mediated synthesis of (1S)-1-amino-2,2-dimethylcyclopropane-1-carboxylic acid
A facile and highly stereoselective synthesis of (1S)-1-amino-2,2-dimethylcyclopropane-1-carboxylic acid 4 via pig liver esterase (PLE) catalysed hydrolysis of a prochiral 2,2-dimethylcyclopropane-1,1-diester is described in the present report. Pig liver esterase has been reported to selectively hydrolyse some rigid, prochiral malonic esters [12].
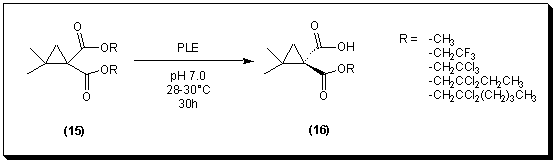
First, the enzymatic asymmetrization of dimethyl 2,2-dimethylcyclopropane-1,1-dicarboxylate 19 was attempted. The diester was prepared by Michael-induced ring closure (MIRC) of the brominated alkylidene malonate 18 [13] with sodium borohydride in methanol. The diester was dispersed in pH 7.0 phosphate buffer at 30�C and enzyme (pig liver esterase) was added. This enzymatic hydrolysis gave the corresponding monoacid 16, but in low enantiomeric excess (ca. 65%).
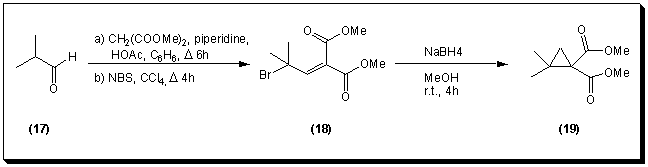
Accordingly, a set of activated esters derived from b-halogenated alcohols was evaluated as prochiral precursors for the PLE hydrolysis. The inductive effect of the halogen atoms would render the corresponding diesters more reactive towards enzymatic hydrolysis. The diesters were prepared by double saponification of dimethyl 2,2-dimethylcyclopropane-1,1-dicarboxylate 19 with sodium hydroxide at room temperature for five days. The resulting 2,2-dimethylcyclopropane-1,1-dicarboxylic acid 20 [14] was then esterified with a b-halogenated alcohol in dichloromethane in the presence of dicyclohexylcarbodiimide (DCC) and 4-(N,N-dimethylamino)pyridine (DMAP) [15].
Only the hydrolysis of bis(2,2,2-trifluoroethyl)-2,2-dimethylcyclopropane-1,1-dicarboxylate 21 with pig liver esterase succeeded, resulting in (1R)-2,2-dimethyl-1-(2,2,2-trifluoroethoxycarbonyl)-cyclopropane-1-carboxylic acid 22 in 62% yield after 30 hours. The enantiomeric excess (>95%) of the monoacid was determined by chiral GC (cyclodextrose capillary column) after esterification of the carboxylic acid group with an excess of diazomethane. These results were supported by 1H-NMR measurements (500 MHz) using (R)-Pirkle's chiral alcohol.
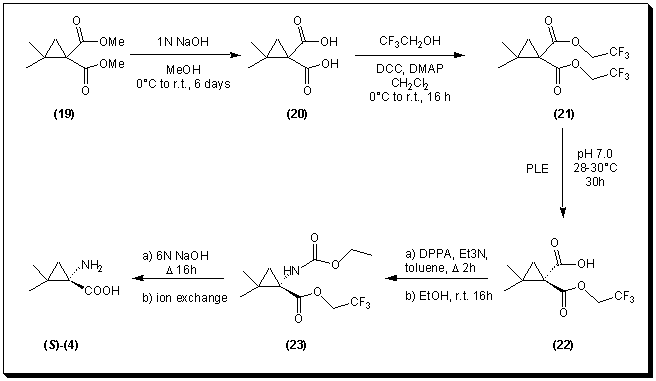
The next step was the Curtius rearrangement of (1R)-2,2-dimethyl-1-(2,2,2-trifluoroethoxycarbonyl)-cyclopropane-1-carboxylic acid 22 with diphenylphosphoroazidate (DPPA) [16]. After work-up with ethanol, this reaction afforded the optically active carbamate (1S)-2,2,2-trifluoroethyl 2,2-dimethyl-1-(N-ethoxycarbonylamino)-cyclopropane-1-carboxylate 23.
Finally, hydrolysis of the carbamate 23 with 6N sodium hydroxide under reflux and purification through cationic ion exchange resin gave (1S)-1-amino-2,2-dimethylcyclopropane-1-carboxylic acid 4. The absolute stereochemistry of the compound obtained was assessed by direct comparison to reported data of its optical rotation. The enantiomeric excess (> 98%) was determined by analytical reverse phase HPLC after derivatization with (R)-Mosher's acid chloride.
[1] |
C. Cativiela and M. Diaz-de-Villegas. Tetrahedron: Asymmetry 11 (2000) 645-732. |
[2] |
|
[3] |
D. Adams, S. F. Yang. Proc. Natl. Acad. Sci. U.S.A., 1979, 76, 170-176. |
[4] |
|
[5] |
|
[6] |
|
[7] |
|
[8] |
R. Chinchilla, C. Najera, S. Garcia-Granda, A. Menendez-Velazquez. Tetrahedron Lett., 1993, 34, 5799-5802. |
[9] |
A. Fadel. Synlett., 1993, 7, 503-505. |
[10] |
C. J. Easton, N. L. Fryer, A. J. Ivory, E. R. T. Tiekink. J. Chem. Soc. Perkin I, 3725-3729 (1998). |
[11] |
M. Kirihata, A. Sakamoto, I. Ichimoto, H. Ueda, M. Honma. Agric. Biol. Chem., 1990, 54(7), 1845-1846. |
[12] |
|
[13] |
R. Verhe, N. De Kimpe, L. De Buyck, D. Courtheyn, N. Schamp. Bull. Soc. Chim. Belg., 1977, 86, 55-63. |
[14] |
J. Bus, H. Steinberg, Th. J. De Boer. Recl. Trav. Chim. Pays-Bas, 1972, 91 (5), 657-666. |
[15] |
|
[16] |
N. De Kimpe, M. Boeykens, K. Abbaspour Tehrani. J. Org. Chem., 1994, 59, 8215-8219. |
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0024 as the message subject of your e-mail.