Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0025]
A Convenient Synthesis of cis-3-Hydroxy-L-Proline
Surajit Sinha, Santosh Tilve+ and Srinivasan Chandrasekaran*
Department of Organic Chemistry, Indian Institute of Science, Bangalore-560012, India.
+ SERC Visiting Fellow on leave from Goa University.
Tel: +91-80-3092404; Fax: +91-80-3600529; E-mail: [email protected]
Received: 25 July 2000 / Uploaded: 29 July 2000
Abstract: A simple synthesis of cis-3-hydroxy-L-proline has been achieved starting from b -alanine making use of Sharpless asymmetric epoxidation as a key step in the synthesis.
cis-3-Hydroxy-L-proline is a relatively rare b -hydroxy-a -amino acid, which has been found as a component of the antibiotic teleomycin.1 In connection with the synthesis of the natural product "cyclothialidine"2, we required to synthesize the optically pure cis-3-hydroxy-L-proline in good chemical yield.
Several syntheses of cis-3-hydroxy-L-proline have been reported. Apart from the enzymatic resolution3 of cis- and trans-3-hydroxy proline, a variety of approaches to the synthesis of this amino acid are reported in the literature with varying degree of success and limitations.4 Most of these utilized enzymatic methods or chiron approach to get the optically pure product.
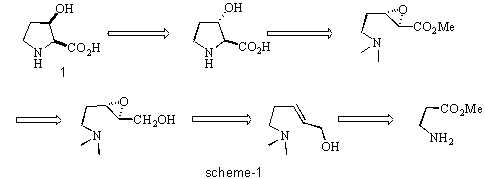
We decided to study a new approach to the asymmetric synthesis of cis-3-hydroxy-L- proline 1 using Sharpless asymmetric epoxidation as a key step in the synthetic strategy. A retrosynthetic analysis of this approach is depicted in scheme-1

Cis-3-hydroxy proline can be derived from trans-3-hydroxy proline which in turn can be formed by an intramolecular cyclisation of an amino epoxide. The chiral epoxide can
be generated by Sharpless epoxidation of a suitable allylic alcohol. The allylic alcohol in turn can be derived from b -alanine by straightforward synthetic manipulations.
The synthetic route was started with the readily available and inexpensive b -alanine. A suitable protective group for the amino group was needed which will be stable to Sharpless epoxidation conditions later in the sequence. It is well documented in the literature5 that the Boc or Cbz protected amines usually lead to the formation of cyclic urethane derivatives under these conditions. Various type of N-protecting groups that would encourage cyclization through nitrogen namely N-tosyl, N-trityl, and even unprotected derivatives have been tried but in all the cases the reaction leads to a complex mixture of products. All the problems are taken care of when nitrogen is protected both with tosyl as well as benzyl group.6
The synthetic route adopted to achieve the synthesis of 1 is presented in scheme-2. The methyl ester of b -alanine was readily converted to the N-protected methylester 2 which on reduction with lithium aluminium hydride in ether (0oC) provided the alcohol 3 in quantitative yield. The alcohol 3 was then oxidized with Dess-Martin periodinane to afford the aldehyde 4 in 97% yield while the Swern oxidation of the same compound gave the product only in 69% yield. The aldehyde 4 was allowed to react with triethylphosphono acetate7 and the trans olefin 5 was obtained in 89% yield. Initially the reduction of the unsaturated ester 5 was tried with lithium aluminium hydride in ether but it led to a mixture of allylic alcohol 6 and the corresponding saturated alcohol. However when the reduction of 5 was carried out with AlH3, generated in situ, the allylic alcohol 6 was the only product obtained in quantitative yield.
Now the stage is set for carrying out Sharpless asymmetric epoxidation. The allyl alcohol 6 was treated with titanium tetraisopropoxide, tert-butyl hydroperoxide, and L-(+)-diethyl tartarate8 and the epoxy alcohol 7 was obtained in 92% yield with 98% ee. The enantiomeric excess was estimated by NMR shift reagent [Eu(hfc)3] experiments.
Initially the direct oxidation of the epoxy alcohol 7 to the epoxy acid 8 with RuCl3/NaIO49 was carried out but the yield obtained in this reaction was generally poor (45% of corresponding methyl ester). Therefore the epoxy alcohol 7 was first oxidized to the aldehyde with Dess-Martin periodinane followed by oxidation with Ag2O to get the epoxy carboxylic acid 8 (91%). Compound 8 was converted to its methyl ester 9 on treatment with diazomethane in high yield. NMR experiments with chiral shift reagent [Eu(hfc)3] revealed the product 9 to have optical purity of 98% ee.

It was then decided to deprotect the N-benzyl group which then can be cyclized on treatment with K2CO3/MeOH. Unfortunately reaction of compound 9 with Pd/C/H2 did not effect the hydrogenolysis as desired. Even under forcing condition at higher hydrogen pressure the hydrogenolysis could not be accomplished efficiently. The next strategy was to remove the tosyl protective group of 9 with Na/napthalene.6 Here again the reaction was not very clean. Finally detosylation of 9 was carried out successfully by reacting it with Mg/MeOH.10 Under these conditions not only did the tosyl group undergo cleavage but it underwent also in situ cyclization to give the N-benzylated 3-hydroxy-L-proline 10 (33% overall yield from b -alanine) .The 1H NMR of 10 did not give any indication as to whether it was the cis- or the trans- isomer. However it was found to be a single diastereomer with 97% ee as confirmed by NMR chiral shift reagent [Eu(hfc)3] experiment as well as HPLC analysis on a chiral column [cyclodex a -pm, solvent: 10% water containing 1% tetraethyl ammonium acetate buffer in MeOH], flow rate 0.4, retention time 5.99 min]. For purposes of characterization compound 10 was converted to the corresponding para-toluene sulfonate salt and in the 1H NMR the a -H appeared as a clear doublet at d 3.18 with a coupling constant of ~1.5 Hz indicating it to be the trans product. Compound 14 on hydrogenolysis (Pd/C/H2) followed by saponification gave a residue which was purified by resin bed column chromatography (elution with 1.5-2M aq. NH3 solution) to afford 15 as a white solid. The melting point (228-236� C) (lit.3 228-235� C) and optical rotation [a ] = -18.3� {lit.11 [a ] = -18.8� } of compound 15 compared with the reported values of trans-3-hydroxy-L-proline. Its optical purity was found to be >97% ee.
The trans-3-hydroxy-L-proline has already been converted to cis-3-hydroxy-L-proline 111 involving Mitsunobu reaction and therefore this constitutes a formal total synthesis of cis-3-hydroxy-L-proline 1.
References
1. Sheehan, J. C.; Whitney, J. G. J. Am. Chem. Soc. 1962, 84, 3980.
2. Nakada, N.; Shimada, H.; Hirata, T.; Aoki, Y.; Kamiyama, T.; Watanabe, J.; Arisawa, M.
Antimicrobial Agents and Chemotherapy, 1993, 37, 2656.
3. a) Morita, K.; Irreverre, F.; Sakiyama, F.; Witkop, B. J. Am. Chem. Soc. 1963, 85, 2832;
b) Sheehan, J.C.; Whitney, J. G. J. Am. Chem. Soc. 1963, 85, 3863; c) Wolff, III, J. S.;
Ogle, J. D.; Logan, M. A. J. Biol. Chem., 1966, 241, 1300.
4. a) Cooper, J.; Gallagher, P.T.; Knight, D.W., J. Chem. Soc., Perkin Trans. 1 1993, 1313.
b) Jurczak, J.; Prokopowicz, P.; Golebiowski, A. Tetraheron Lett., 1993, 7107.
c) Sundram, H.; Golebiowski, A.; Johnson, C.R. Tetraheron Lett., 1994, 6975.
d) Sibi, M.P.; Christensen, J.W. Tetraheron Lett. 1995, 6213.
e) Dell�Uomo, N.; Di Giovanni, M.C.; Misiti, D.; Zappia, G.; Monache, G.D. Tetraheron :
Asymmetry 1996, 7 181.
f) Gotschi, E.; Jenny, C-J.; Reindl, P.; Ricklin, F. Helv. Chim. Acta. 1996, 79, 2219.
5. Baldwin, J.E.; Flinn, A. Tetraheron Lett. 1987, 3605.
6. Adams, C.E.; Walker, F.J.; Sharpless, K.B. J. Org Chem. 1985,50,422.
7. Villieras, J.; Rambaudet, M.; Graff, M. Tetraheron Lett. 1985,53.
8. Pfenninger, A. Synthesis 1986, 89.
9. Carlsen, P.J.; Katsuki, T.; Martin, V.S.; Sharpless, K.B.J. Org. Chem. 1981, 46, 3937.
10. Alonso, D.A.; Andersson, P.G. J. Org. Chem., 1998, 63, 9455.
11. Herdeis, C.; Hubmann, H.P. Tetraheron: Asymmetry 1994, 5, 119.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0025 as the message subject of your e-mail.