Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0028]
Palladium(II) Supported by Hydrotalcite[Pd(II)-Hydrotalcite]-Catalyzed Selective Oxidation of Alcohols Using Molecular Oxygen
Nobuyuki Kakiuchi, Takahiro Nishimura, Masashi Inoue and Sakae Uemura*
Received: 8 July 2000 / Uploaded: 29 July 2000
Novel heterogenized Pd catalyst, palladium supported by hydrotalcite[Pd(II)-hydrotalcite], has been synthesized. The catalyst is found to be effective for the oxidation of a wide range of alcohols using molecular oxygen as a sole oxidant. In this catalytic system, various alcohols are readily converted to the corresponding aldehydes or ketones selectively in high to excellent yields. It is noteworthy that the catalyst is also applicable to the oxidation of unsaturated alcohols such as geraniol and nerol without any isomerization of an alkenic part. Another characteristic property of this heterogeneous catalyst is that a shape selectivity depending on the structure of alcohols is observed in some extent. The catalyst has also advantages such as ease of handling, easy preparation, and reusability for several times without appreciable loss of the catalytic activity.
Introduction
The increasing environmental and economical concerns let chemists to develop the clean and high-performance catalytic reaction. Especially, much attention has been paid for the heterogeneous or heterogenized catalysts [1,2] because of their unique properties such as reusability and molecular recognition effect. For example, the application of these catalysts for aerobic oxidation of alcohols is an interesting target for many chemists. Immobilization of transition metal salts to various kinds of supports such as activated carbon, alumina, silica, clays and polymers is an important method to produce such effective heterogeneous catalysts. For recent examples, the aerobic oxidation of alcohols was successfully carried out using heterogenized catalysts such as polymer- or mesoporous solid MCM-41-supported TPAP (tetrapropylammonium perruthenate) [3,4] and TiO2-supported palladium cluster [5b,c]. Ruthenium doped hydrotalcites (layered basic clay minerals) were also reported as efficient heterogeneous catalysts for the oxidation of alcohols under O2[6]. Recently, we reported the oxidation of alcohols using a homogeneous palladium catalyst under atmospheric pressure of oxygen [7], and also succeeded in the immobilization of the homogeneous palladium catalyst on hydrotalcite (Mg6Al2(OH)16CO3 · 4H2O) and its application for aerobic oxidation of alcohols [8]. In this paper we describe details of this aerobic oxidation using Pd(II)-hydrotalcite as catalyst [9].
Results and Discussion
Preparation
and Characterization of Pd(II)-Hydrotalcite.
Pd(II)-hydrotalcite
was prepared by mixing Pd(OAc)2 (1.67 mmol),
pyridine (4.18 mmol) and hydrotalcite (10.0 g) in toluene (100 mL) at 80°C for 1 h,
followed by filtration, washing, and drying under vacuum at room temperature. In this
process Pd(OAc)2 · (py)2 complex [10] was initially formed and then it was
adsorbed on the hydrotalcite support. The Pd content in the Pd(II)-hydrotalcite was 0.16
mmol g-1 estimated by inductively
coupled plasma (ICP) atomic emission spectrometry. Hydrotalcite has a layered structure
consisting of positively charged brucite-like layers and negatively charged counter ions
and water molecules located in the interlayers [11]. Thus, we first investigated the
spacing between layers of newly prepared Pd(II)-hydrotalcite. Spacing (d003) of both commercially available hydrotalcite and the
Pd(II)-hydrotalcite was estimated to be almost the same by X-ray diffraction (XRD)
analysis. These results suggest that the palladium salt does not exist between brucite
layers by an anion exchange. The possibility of the substitution of any cations in brucite
layers by the palladium salt is also low because of a stable structure of the brucite. So,
we postulated that the palladium salt is located on the external surface of thin
plates-like crystals of hydrotalcite (not between layers). For the purpose of confirming
our assumption and disclosing the dispersion state of the palladium salt, the
Pd(II)-hydrotalcite was analyzed by transmission electron microscopy (TEM). At low
magnification, a number of small particles were observed on the plates-like crystals of
the hydrotalcite. However, because of severe dehydration of hydrotalcite by irradiation of
electron beam, the high magnification image of surface morphology of Pd(II)-hydrotalcite
could not be obtained. Next, we attempted to investigate the presence of pyridine in
Pd(II)-hydrotalcite. Elemental analysis showed that 0.35% of N atom was contained in
Pd(II)-hydrotalcite, which was quite close to a theoretical percentage of nitrogen (0.45%
calculated from the result of ICP atomic emission analysis, assuming that Pd atoms exist
in the Pd(OAc)2 · (py)2 form in Pd(II)-hydrotalcite). Further evidence of the
existence of pyridine in Pd(II)-hydrotalcite was obtained by thermogravimetry / mass
spectrometry (TG/MS) analysis. MS peak of pyridine (m/z = 79) desorbed from
Pd(II)-hydrotalcite was observed at around 160°C [12]. We also carried out control
experiments to rule out the possibility of adsorption of pyridine on hydrotalcite; that
is, another sample was prepared by treating hydrotalcite only with pyridine in toluene and
by washing the resultant solid in the same way as in the preparation of
Pd(II)-hydrotalcite. In this sample, pyridine was not detected by elemental analysis and
TG/MS. These results suggest that pyridine exists as a ligand on palladium in
Pd(II)-hydrotalcite. Thus, we concluded that the palladium-pyridine complex was
immobilized on the external surface of brucite layers of Pd(II)-hydrotalcite [13].
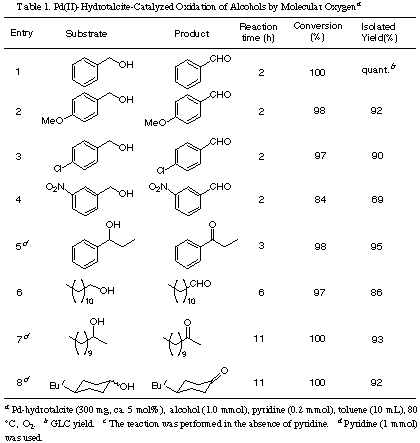
Pd(II)-Hydrotalcite-Catalyzed Oxidation of Alcohols.

Oxidation of Unsaturated
Alcohols.
The oxidation of
alkenic alcohols using both Pd(II)-hydrotalcite system and Pd(OAc)2 / pyridine / MS3A system (homogeneous catalytic system; abbreviated as
MS-system) [7b] was performed for comparison, and the results were summarized in Table 2.
In the oxidation of alkenic alcohols, a quite excess of pyridine (25 times as much as a
standard condition) was necessary to complete the reaction. The excess of pyridine may
prevent the complexation of Pd(II)-intermediates with an alkenic moiety which might
accelerate the reduction of Pd(II) [15]. When the oxidation of cinnamyl alcohol using both
catalytic systems was compared, Pd(II)-hydrotalcite system showed a slightly higher
activity than MS-system to give cinnamaldehyde in 95% isolated yield (Entry 1). In the
oxidation of a secondary allylic alcohol, a similar trend was observed (Entry 2). Alkenic
alcohols such as 10-undecen-1-ol could also be smoothly oxidized to give the corresponding
aldehyde in good yield without affecting the alkenic moieties (Entry 3). Next, the
oxidation of nerol [(Z)-isomer] and geraniol [(E)-isomer] was carried out.
Using MS-system, the yields of aldehydes were low (39 and 56%, respectively) and E/Z
ratios of the products were seriously disturbed (E/Z ratios were 31/69 and
63/37, respectively) [16]. On the other hand, Pd(II)-hydrotalcite-catalyzed reaction
smoothly proceeded to give the corresponding aldehydes in 89% and 91% yields
without any geometrical isomerization (E/Z ratios were 6/94 and 96/4)
(Entries 4 and 6). Further, under these conditions reaction rates dramatically increased
using Pd(II)-hydrotalcite. It is well known that a Pd(II) species is generally not the
effective catalysts for oxidation of allylic alcohols because of its strong complexation
with unsaturated carbon-carbon bonds [9a]. Recently, such drawbacks in palladium chemistry
have been overcome and some good examples have been reported on palladium-catalyzed
oxidation of allylic alcohols into the corresponding aldehydes or ketones [5,9e,f]. Our
results also provide a good method for the selective aerobic oxidation of allylic alcohols
[17]. Although the reason for preservation of the E/Z geometry in products
in the case of Pd(II)-hydrotalcite system was not yet clear, the complexation of the
palladium species with the alkenic part may be inhibited because of the steric bulkiness
of hydrotalcite surface.

Recycle of the Catalyst.

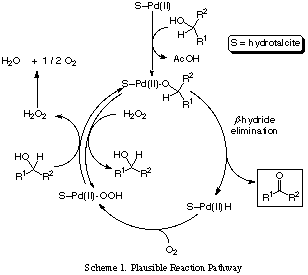
Pd(II)-hydrotalcite was newly prepared by simple operation from commercially available hydrotalcite, Pd(OAc)
2 and pyridine. This novel clay compound efficiently catalyzed the aerobic oxidation of various kinds of alcohols including unsaturated ones. This catalyst could be reused for several times keeping its catalytic activity.
Materials. Pd(OAc)2 was purchased from Wako Pure Chemical Ind., Ltd., and used without further purification. Hydrotalcite (Mg6Al2(OH)16CO3 · 4H2O, brand name KYOWAAD®500) was kindly supplied by Kyowa Chemical Ind., Ltd. Pyridine was purchased and used without further purification. Toluene was distilled before use. MS3A powder was commercially available from Nacalai Tesque Chemical Co., Inc., which was activated by calcination (by a gas burner) just before use
General Procedure for Preparation and Characterization of Pd(II)-Hydrotalcite. To a mixture of Pd(OAc)2 (375 mg, 1.67 mmol) and toluene (100 mL) in a 200 mL two-necked flask was added pyridine (331 mg, 4.18 mmol) at 80°C. The brown suspension turned to a yellow-white suspension when pyridine was added. Hydrotalcite (KYOWAAD®500, 10.0 g) was added and the mixture was stirred vigorously for 1 h at 80°C. Then, the resulting slurry was cooled to 0°C, followed by filtration and washing with diethyl ether (2 × 20 mL). The resulting solid was dried under vacuum at room temperature to give ca. 10 g of a light-yellow powder of Pd(II)-hydrotalcite. The Pd content in the Pd(II)-hydrotalcite was 0.16 mmol g-1 estimated by ICP atomic emission analysis. Elemental analysis; Pd(II)-hydrotalcite; N, 0.35% [Calcd. N, 0.45% calculated from the result of ICP emission analysis, assuming that Pd atoms exist in the Pd(OAc)2 · (Py)2 form in Pd(II)-hydrotalcite]. The values of atomic spacing d003 were estimated by XRD analysis as follows: commercially available hydrotalcite, 7.81Å; Pd(II)-hydrotalcite, 7.75Å. Pd(II)-hydrotalcite* was prepared by a similar method as described above using a half scale of Pd(OAc)2 (188 mg), pyridine (165 mg), and toluene (50 mL) with 10 g of hydrotalcite. The Pd content in the Pd(II)-hydrotalcite* was 0.092 mmol g-1 estimated by ICP atomic emission analysis.
General Procedure for Pd(II)-Hydrotalcite or Pd(II)-Hydrotalcite*-Catalyzed Oxidation of Alcohols Using Molecular Oxygen. A typical experimental procedure is as follows: to a suspension of the Pd(II)-hydrotalcite (300 mg, ca. 5 mol% as Pd) or Pd(II)-hydrotalcite* (600 mg, ca. 5 mol% as Pd) in toluene (6 mL) in a 20 mL two-necked flask was added pyridine (0.2-5 mmol) and the mixture was stirred. Then, oxygen gas was introduced into the flask from an O2-balloon under atmospheric pressure, and the mixture was heated to 80°C for ca. 10 min. Next, an alcohol (1 mmol) in toluene (4 mL) was added and the mixture was stirred vigorously for 2 h (or appropriate time) at 80°C under oxygen. After the reaction, the catalyst was separated by filtration through a glass filter. Removal of the solvent from the filtrate under the reduced pressure left an oily residue which was subjected to column chromatography (Merck silica gel 60; eluents, hexane-diethyl ether) to give a product. Products obtained were determined by 1H and 13C NMR and GC/MS.
General Procedure for Pd(OAc)2 / Pyridine / MS3A System-Catalyzed Oxidation [7b] of Unsaturated Alcohols Using Molecular Oxygen. An improved procedure for the oxidation of unsaturated alcohols using homogeneous catalyst is as follows: to a suspension of the Pd(OAc)2 (11.2 mg 0.05 mmol) in toluene (4 mL) in a 20 mL two-necked flask were added pyridine (5 mmol) and MS3A (500 mg), and the mixture was stirred at room temperature. Then, oxygen gas was introduced into the flask from an O2-balloon under atmospheric pressure. Next, an alcohol (1 mmol) in toluene (6 mL) was added at room temperature and the mixture was heated to 80°C and stirred vigorously for appropriate time under oxygen.
General Procedure for Recycling of the Catalyst. First run of the oxidation of benzyl alcohol catalyzed by Pd(II)-hydrotalcite or Pd(II)-hydrotalcite* was performed using the same procedure described above. Recovered Pd(II)-hydrotalcite or Pd(II)-hydrotalcite* was washed with diethyl ether (2 × 20 mL) and dried under vacuum at room temperature before use for the next run. The method of the test for Pd-leaching was as follows; the usual oxidation of benzyl alcohol was allowed to proceed for 30 min, and the catalyst was filtered at 80°C. Then, the filtrate containing the product benzaldehyde and the unreacted benzyl alcohol was stirred under O2 at 80°C. The reaction was monitored with GLC using cyclododecane as an internal standard.
Acknowledgment
The authors gratefully thank to Kyowa Chemical Ind., Ltd. for the gift of hydrotalcite (Mg6Al2(OH)16CO3 · 4H2O, brand name KYOWAAD®500) available to this study. We also thank Dr. Tatsuya Takeguchi (Kyoto Univ.) for his help in CP-MAS 13C-NMR analysis and Dr. Shinji Iwamoto (Kyoto Univ.) for his help in ICP atomic emission analysis. T. N. gratefully acknowledges a Fellowship of the Japan Society for the Promotion of Science for Young Scientists.
References and Notes
1 For example, see: M. Balogh and P. Laszlo, "Organic Chemistry Using Clays," Springer-Verlag, New York (1993); R. L. Augustine, "Heterogeneous Catalysis for the Synthetic Chemist," Marcel Dekker, New York (1996); R. A. Sheldon and R. S. Downing, Appl. Catal. A, 189, 163 (1999).
2 Our previous reports about organic reactions using heterogeneous catalysts: J. Tateiwa, H. Horiuchi, K. Hashimoto, T. Yamauchi and S. Uemura, J. Org. Chem., 59, 5901 (1994); J. Tateiwa, T. Nishimura, H. Horiuchi and S. Uemura, J. Chem. Soc., Perkin Trans. 1, 1994, 3367; J. Tateiwa, H. Horiuchi and S. Uemura, J. Chem. Soc., Perkin Trans. 2, 1995, 2013; J. Tateiwa, H. Horiuchi and S. Uemura, J. Org. Chem., 60, 4039 (1995); J. Tateiwa, E. Hayama, T. Nishimura and S. Uemura, Chem. Lett., 1996, 59; J. Tateiwa, E. Hayama, T. Nishimura and S. Uemura, J. Chem. Soc., Perkin Trans. 1, 1997, 1923; T. Nishimura, S. Ohtaka, A. Kimura, E. Hayama, Y. Haseba, H. Takeuchi and S. Uemura, Appl. Catal. A, 194-195, 415 (2000).
3 B. Hinzen, R. Lenz and S. V. Ley, Synthesis, 1998, 977.
4 A. Bleloch, B. F. G. Johnson, S. V. Ley, A. J. Price, D. S. Shephard and A. W. Thomas, Chem. Commun., 1999, 1907.
5 a) K. Kaneda, M. Fujii and K. Morioka, J. Org. Chem., 61, 4502 (1996). b) K. Kaneda, Y. Fujie and K. Ebitani, Tetrahedron Lett., 38, 9023 (1997). c) K. Ebitani, Y. Fujie and K. Kaneda, Langmuir, 15, 3557 (1999).
6 K. Kaneda, T. Yamashita, T. Matsushita and K. Ebitani, J. Org. Chem., 63, 1750 (1998); T. Matsushita, K. Ebitani and K. Kaneda, Chem. Commun., 1999, 265.
7 a) T. Nishimura, T. Onoue, K. Ohe and S. Uemura, Tetrahedron Lett., 39, 6011 (1998). b) T. Nishimura, T. Onoue, K. Ohe and S. Uemura, J. Org. Chem., 64, 6750 (1999). Application of a similar catalytic system to the oxidative reaction of tert-cyclobutanols: c) T. Nishimura, K. Ohe and S. Uemura, J. Am. Chem. Soc., 121, 2645 (1999).
8 T. Nishimura, N. Kakiuchi, M. Inoue and S. Uemura, Chem. Commun., 2000, 1245.
9 As examples of palladium-catalyzed aerobic oxidation, see for example: a) T. F. Blackburn and J. Schwartz, J. Chem. Soc., Chem. Commun., 1977, 157. b) M. Hronec, Z. Cvengrosová and J. Kizlink, J. Mol. Catal., 83, 75 (1993). c) E. Gómez-Bengoa, P. Noheda and A. M. Echavarren, Tetrahedron Lett., 35, 7097 (1994). d) G. Noronha, P. M. Henry, J. Mol. Catal. A, 120, 75 (1997). e) K. P. Peterson and R. C. Larock, J. Org. Chem., 63, 3185 (1998). f) G.-J. ten Brink, I. W. C. E. Arends, R. A. Sheldon, Science, 287, 1636 (2000).
10 Pd(OAc)2 · (Py)2 complex was prepared by treatment of Pd(OAc)2 with pyridine, see: a) S. V. Kravtsova, I. P. Romm, A. I. Stash and V. K. Belsky, Acta Crystallogr., C52, 2201, (1996). b) T. A. Stephenson, S. M. Morehouse, A. R. Powell, J. P. Heffer and G. Wilkinson, J. Chem. Soc., 1965, 3632.
11 F. Cavani, F. Trifirò and A. Vaccari, Catal. Today, 11, 173 (1991).
12 Pd(OAc)2 · (Py)2 complex decomposed at 185°C; see ref. 10b.
13 Although it is not clear what kinds of interactions let palladium-pyridine complex be immobilized on hydrotalcite, there is a possibility of ionic bonding between Pd(II)-pyridine complex and hydroxyl groups on the surface of the hydrotalcite. A short comment about this kind of interaction was stated (See ref. 5c).
14 The presence of an excess pyridine (to palladium) is essential for maintaining the oxidation state of Pd(II). The absence of additional pyridine might cause the formation of Pd(0) species, resulting in a low catalytic activity. Actually, the color of the powder of the recovered catalyst changed from yellow-white to gray after the reaction without a further addition of pyridine, while such color change was not observed in the reaction with additional pyridine.
15 We suppose that the present reaction proceeds in a similar pathway to the homogeneous catalytic system as previously described (See, ref. 7b). Thus, pyridines coordinate to Pd(II) as ligands and stabilize the Pd(II)-hydride species to avoid the reductive elimination of HX from HPdX species to give Pd(0).
16 In our previous report (ref. 7b), we
failed the oxidation of geraniol in Pd(OAc)2 / pyridine / MS3A system.
However, this limitation could be overcome by slightly improving the
procedure. See experimental section.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0028 as the message subject of your e-mail.