Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0031]
(S)-5-(Tosylmethyl)-2-pyrrolidinone: A New Chiral b -Amidosulfone for a Short Asymmetric Synthesis of Indolizidines
Tomas Abellan, Diego Alonso, Jesus Casas, Ana Costa, Carmen Najera*, Jose M. Sansano and Montserrat Varea.
Departamento de Quimica Organica, Universidad de Alicante. E-03080 Alicante, Spain. Tel. +34-96-5903728, Fax +34-96-5903549, E-mail: [email protected]
Abstract: The synthesis of (S)-5-(tosylmethyl)-2-pyrrolidinone from (S)-pyroglutaminol [(S)-2-(hydroxymethyl)-2-pyrrolidinone] in three steps and its applications into the synthesis of indolizidines is described in this communication.
Keywords: Asymmetric synthesis, Sulfones, Indolizidines, Dianions, Alkylation.
Received: 18 July 2000 / Uploaded: 29 July 2000Introduction
Indolizidine alkaloids are characterised by fused six- and five-membered ring with a nitrogen atom at the ring fusion. These natural products are widely distributed and have been isolated from plants, animals and some fungi. They are toxic to animals, for example, inhibiting intestinal hydrolases, they are also mutagenic but especially polyhydroxyindolizidines have demonstrated activity against AIDS virus HIV and against some carcinogenic cells[1].
Several synthetic methodologies and total synthesis have been developed in order to form this bicyclic heterocycle with the suitable stereochemistry on its substituted carbon atoms[1][2]. In this communication we report the preparation of a new chiral b -amidosulfone which is a useful building block in the diastereoselective synthesis of indolizidines.
Discussion
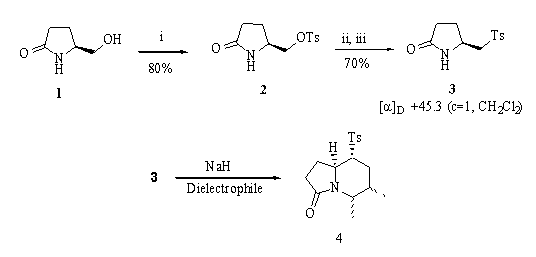
Chiral sulfone 3 was prepared from (S)-pyroglutaminol[3] 1 in a three steps sequence in 56% overall yield. Alcohol 1 was transformed into the tosylate 2 (80% yield) which underwent nucleophilic substitution with p-methylthiophenol sodium salt in refluxing acetonitrile. Finally, sulfone 3 was generated by oxidation of the resulting thioether using Oxonea in methanol (Scheme).
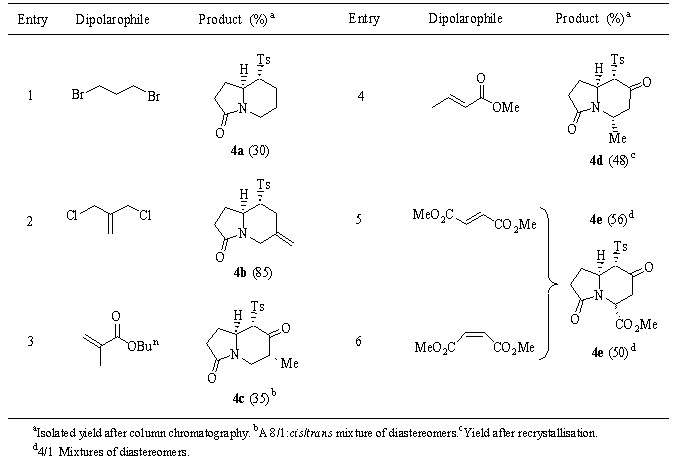
The reaction of the dianion of b -amidosulfone 3, generated with 2.5 equiv. of sodium hydride in DMF at room temperature, with dielectrophiles afforded very interesting indolizidines (Scheme and Table) which incorporate strategically the substituents depending on the dielectrophile employed. The cyclisation step occurred with high diastereoselectivity with the tosyl group settled at the ecquatorial position. Yields are, in general, moderate therefore a very high chemical yield was achieved (85%) when 3-chloro-2- (chloromethyl)-1-propene was the electrophile. Another dialkylation reactions drove to low yields due to b -eliminations and polymerisations as occur in entries 1 and 3 (Table), respectively[4].

Reagents: i. TsCl, DMAP (cat), Et3N, CH2Cl2. ii. p-TolSNa, MeCN, 80?C. iii. Oxonea , MeOH/H2O.
iv. NaH, DMF, dielectrophile, rt.
Table.
Conclusion
We have elaborated a versatile chiral building block, (S)-5-(tosylmethyl)-2-pyrrolidinone, able to react with dielectrophiles affording, in only one step, the chiral skeleton of indolizidine alkaloids.
Experimental Part
The full experimental section of precursors will be described elsewhere. General procedure for the dialkylation reaction of compound 3 furnishing indolizidine precursor 4b is the following:
To a suspension of sodium hydride (95%, 19 mg, 0.75 mmol) in DMF (2 ml) was added a solution of sulfone 3 (86 mg, 0.34 mmol) in DMF (2.5 ml) and the resulting mixture was stirred for 1h at room temperature. 3-Chloro-2-(chloromethyl)-1-propene (0.048 ml, 0.41 mmol) and sodium iodide (153 mg, 1.02 mmol) was then added and stirring continued for 16h at the same temperature. A saturated aqueous solution of ammonium chloride (4 ml) and ethyl acetate (3x5 ml) were added and the organic phase was again washed with water (5 ml), dried (Na2SO4) and evaporated in vacuo affording the crude product which was purified by column chromatography (SiO2) eluting with mixtures of hexane and ethyl acetate giving 4b (96 mg, 85%) as colourless solid.
M.p.: 161-162�C from hexane/ethyl acetate.
TLC: 0.53 (ethyl acetate).
IR (KBr): 3062, 1687, 1599, 1290 and 1144 cm-1.
1
H NMR (300 MHz, CDCl3): 2.36-2.61 [m with s at 2.49, 9H, CH3Ar, (CH2)2CO and CH2CHS); 2.95 (ddd, J = 12.2, 10.4 and 4.3, 1H, CHS); 3.20 (d, J = 14.0 Hz, 1H, HCHN), 3.81 (dt, J = 10.4, 7.3 Hz, 1H, CHN); 4.49 (d, J = 14.0 Hz, 1H, HCHN); 4.85, 4.99 (2s, 2H, CH2=C); 7.72 and 7.77 (2d, J = 7.9 Hz, 4H, ArH).13
C NMR (75 MHz, CDCl3): 21.63 (CH3Ar), 24.85, 30.12, 33.55 [(CH2)2CO, CH2CHS], 45.18 (CH2N), 55.66 (CHS), 66.65 (CHN), 113.72, 134.07 (C=CH2), 128.78, 130.07, 136.75, 145.53 (ArC) and 173.16 (CO).MS (EI): 304 (M++1, 1%), 1500 (22), 149 (100), 148 (84), 134 (13), 91 (13) and 65 (10).
References and Notes
[1] a) Comprehensive Natural Products Chemistry. Eds. Barton, D.H.B.; Nakanishi, K. Elsevier Science, Oxford, 1999, vol. 4, p. 25. b) Dewick, P. M. Medicinal Natural Products. Wiley, 1998, p. 289. c) Michael, J. P. Indolizidine and quinolizidine alkaloids. Nat. Prod. Rep. 1999, 16, 675.
[2] Some recent references for the synthesis of indolizidines: a) Michael, J. P.; Gravestock, D. Vinylogous uretanes in alkaloid synthesis. Applications to the synthesis of racemic indolizidine 209B and its diastereoisomer (5R*,8S*,8aS*)-(� )-diastereomer, and to (-)-indolizidine 209B. J. Chem. Soc., Perkin Trans. 1 2000, 1919. b) Vicente, J.; Gomez Arrayas, R.; Canada, J.; Carretero, J. C. Stereoselective synthesis of (-)-swainsonine and 1,2-di-epi-swainsonine from g -hydroxy-a ,b -unsaturated sulfones. Synlett, 2000, 53. c) Carretero, J. C.; Gomez Arrayas, R. Stereoselective synthesis of polyhydroxylated indolizidines from g -hydroxy-a ,b -unsaturated sulfones. J. Org. Chem. 1998, 63, 2993. d) Caturla, J. F.; Najera, C. (E)-5-Tosyl-4-pentenamide: a vinyl sulfone for the one pot general synthesis of indolizidine derivatives.Tetrahedron Lett. 1997, 21, 3789.
[3] Obtained from pyroglutamic acid. For other synthetic applications see: Najera, C.; Yus, M. Pyroglutamic acid: a versatile building block in asymmetric synthesis. Tetrahedron:Asymmetry 1999, 10, 2245.
[4] This work was supported by DGICYT (Proyecto n? PB97-0123) and by Generalitat Valenciana (Proyecto GVDOC00-14-02).
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0031 as the message subject of your e-mail.