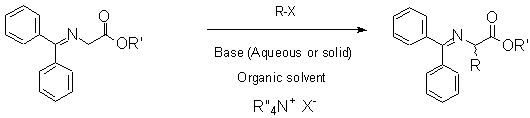
Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0032]
New Chiral Phase Transfer Supported Catalysts
in The Asymmetric Synthesis of a -Amino Acids :
The Importance of a Spacer
Dominique Cahard,* Baptiste Thierry, Jean-Christophe Plaquevent
Universite de Rouen, UPRES-A 6014 et Institut de Recherche en Chimie Organique Fine (IRCOF), Rue Tesniere 76821 Mont Saint Aignan Cedex, France
E-mail: [email protected]
Received: 19 July 2000 / Uploaded: 29 July 2000
Abstract:
Polymer-supported cinchona alkaloid salts with different spacers were used as phase transfer catalysts in the asymmetric C-alkylation of N-diphenyl methylene glycine t-butyl ester for the synthesis of phenylalanine. Various catalysts and alkylation conditions were studied, the best result being 81% ee with cinchoninium iodide bound to polystyrene with a four carbon spacer.
Keywords: a -amino acids, polymer-supported catalysts, asymmetric phase transfer catalysis
The asymmetric synthesis of a -amino acids remains a major challenge in organic chemistry.1 An especially attractive method, introduced first by O�Donnell in 1989,2 uses the liquid/liquid phase transfer catalysed asymmetric alkylation of N-diphenyl methylene glycine t-butyl ester under the influence of N-benzyl cinchona alkaloid salts (first generation, vide infra) as phase transfer catalysts (Scheme 1). Enantiomeric excesses up to 66% were obtained by this method.
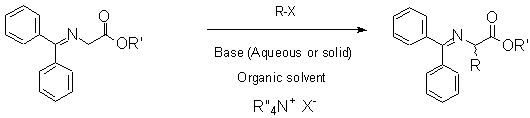
Scheme 1
Three generations of catalysts derived from cinchona alkaloids were successively developed (Figure 1).

Figure 1
The second generation of catalysts, i.e. the N-alkyl O-alkyl cinchona alkaloid salts were reported in 1994,3 also by O'Donnell and coworkers. The best enantiomeric excess obtained with these catalysts was 81%.3 Finally, the third generation of catalysts was described independently by Lygo4 and Corey5 in 1997 in which a 9-anthracenylmethyl group was introduced as a very effective unit for masking the nitrogen face, leading to enantiomeric excesses up to 94%.5
Catalytic asymmetric synthesis of a -amino acids by polymer-supported phase transfer catalysts appears to us a particularly attractive method. Indeed, there are several practical advantages when polymer-supported catalysts are used, i.e. the isolation of product is simplified, the catalyst can easily be recovered by simple filtration and possibly reused. Some examples of polymer-supported phase transfer catalysts have already been described in literature. However, results obtained by such methods are lower than with unsupported catalysts. Indeed, the best result regarding enantioselective alkylation of glycine derivatives using supported cinchona alkaloids salts as phase transfer catalysts is 27% ee.6 The catalyst was prepared by grafting cinchona alkaloids on Merrifield resins via the quinuclidinium nitrogen atom. It is important to note the absence of a spacer between the polymer and the chiral auxiliary in this case. The aim of the present work was to test the role of a possible spacer, since we considered that distancing the chiral moiety from the matrix could enhance asymmetric induction. Thus, we decided to synthesise new families of supported cinchona alkaloids, with a variety of spacers. The synthesis and catalytic activity of these new polymers is described below.
Starting from polystyrene (1% divinylbenzene), we performed lithiation by means of the complex butyllithium / TMEDA. The lithiated polymer reacted with a w-dihalogened (bromo, chloro) alkyl chain, which constitutes the spacer. Then, for reasons of reactivity, we substituted the terminal chlorine for iodine to allow easier substitution on the alkaloid (Scheme 2).

Scheme 2
We focused our strategy on two spacer lenghts, that of four and six carbon atoms. The four alkaloids (quinine, quinidine, cinchonine and cinchonidine) were grafted, giving eight new chiral polymers. When testing these polymers (see Scheme 1, R: benzyl, R�: t-butyl, base: potassium hydroxide, solvent: toluene, room temperature), we obtained the following results (Table 1):
Table 1: Enantioselective alkylation by means of chiral polymer-supported catalysts
Alkaloid |
n |
Reaction time (h) |
Yield (%)a |
ee(%)b (Abs config) |
Cinchonine |
6 |
40 |
61 |
54 (R) |
Cinchonidine |
6 |
96 |
48 |
29 (R) |
Quinine |
6 |
72 |
26 |
6 (R) |
Quinidine |
6 |
72 |
33 |
9 (R) |
Cinchonine |
4 |
12 |
60 |
63 (R) |
Cinchonidine |
4 |
48 |
57 |
6 (R) |
Quinine |
4 |
48 |
43 |
8 (R) |
Quinidine |
4 |
48 |
39 |
4 (R) |
a
After flash chromatography over silica gel (eluent: dichloromethane:triethylamine, 99:1). b determined by HPLC (Chiralcel OD column, 2-propanol:heptane / 0,5:99,5, 1mL/min, 23�C, l = 254 nm) and polarimetry.With the catalysts bearing the six carbon spacer, cinchonine and cinchonidine derivatives gave significant results, 54% and 29% enantiomeric excess, while the catalysts derived from the two other alkaloid diastereomers showed low asymmetric induction. The importance of both the length of the spacer and of the nature of the alkaloid is illustrated in the four carbon spacer series for which only cinchonine gave a good result (63% ee). To our surprise, we always obtained the same major enantiomer whatever the catalyst used, even though cinchonine and cinchonidine are known to behave as pseudoenantiomers,7 in the sense that the two families normally give rise to opposite enantiomers in phase transfer catalysed reactions. This implies that the hydroxy group in the catalyst is not involved to any significant extent in the enantiotopic differentiation of the reaction. By inference, those stereogenic centers of the quinuclidinium that remain invariant through the series must be responsible of asymmetric induction in this case.8 Indeed, by comparaison to unsupported catalysts derived from cinchona alkaloids having a benzyl or antracenylmethyl group, the quinuclidinium nitrogen in polymer supported catalysts bearing the spacer is not as sterically hindered. The best catalyst, that is cinchonine anchored through a four carbon spacer, was further optimized in order to reach still higher ee's. A number of conditions of the reaction (solvent, base, or temperature) were investigated (Table 2).
Table 2: Optimization of reaction conditions
Solvent |
Base |
Temperature (�C) |
Time (h) |
Yield (%) |
ee (%) |
dichloromethane |
aq KOH |
25 |
16 |
60 |
14 |
toluene |
aq KOH |
25 |
12 |
60 |
63 |
toluene |
aq KOH |
0 |
15 |
60 |
81 |
toluene |
solid CsOH |
25 |
1 |
51 |
26 |
toluene |
solid CsOH |
0 |
6 |
48 |
67 |
Toluene appeared to be a better solvent than dichloromethane. Moreover, by cooling the mixture to 0�C, we obtained our best enantiomeric excess to date of 81%. A solid-solid-liquid PTC was also examined with cesium hydroxide as the basic phase in order to minimize the possibility of water in the organic phase, the rate of reaction was dramatically increased and ee slightly reduced, but under such conditions we have the possibility to lower the reaction temperature, which may allow higher enantioselectivity.
Conclusion:
Comparable synthesis with the first generation of catalysts derived from cinchona alkaloids, led to 66% ee.2 Results described herein are non solum much more promising than those obtained with other supported catalysts (ee 27%), sed etiam better than those obtained with unsupported catalysts. These data suggest the great importance of the spacer between the chiral auxiliary and the matrix. Further studies are in progress to improve enantioselectivity and to gain insights into the mechanism of the asymmetric induction and to generalise the use of this familly of polymer supported catalysts to other reactions.
Aknowledgments:
We thank the reseau interregional normand � PUNCH�Orga � for supporting this study, and Pr Dehmlow for helpful discussions about the absence of the pseudoenantiomeric effect.
References and notes:
1- For reviews, see for examples: R.M. Williams, Advances in asymmetric Synthesis, Vol 1, 45-94; R.O. Duthaler, Tetrahedron, 1994, 50, 1539-1650.
2- M.J. O�Donnell, W.D .Bennett, S. Wu, J.Am.Chem.Soc. 1989, 111, 2353-2355.
3- M.J. O�Donnell, S. Wu, C. Hoffman, Tetrahedron, 1994, 50, 4507-4518.
4- B. Lygo, P.G. Wainwright, Tetrahedron Letters, 1997, 38, 8595-8598.
5- E.J. Corey, F. Xu, M.C. Noe, J.Am.Chem.Soc. 1997, 119, 12414-12415.
6- Y. Wang, Z. Zhang, Z. Zhen, J. Meng, P. Hodge, Chinese Journal of Polymer Science, 1998, V16, 356-361.
7- M.J. O�Donnell, F. Delgado, R.S. Pottorf, Tetrahedron, 1999, 55, 6347-6362.
8- The absence of the pseudoenantiomeric effect has previously been observed in the enantioselective synthesis of aziridines: J. Aires de Souza, A.M. Lobo, S. Prabhakar, Tetrahedron Letters, 1996, 37, 3183-3186.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0032 as the message subject of your e-mail.