Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0034]
Copper Mediated Addition of Organo-zinc Halides to N-benzyl Benzenesulfonimine.
David Rosenberg, Jason Kraska, Reuben D. Rieke*
E-mail: [email protected]
Received: 24 July 2000 / Uploaded: 29 July 2000
Abstract: Addition of selected organo-zinc halides to N-phenyl Benzenesulfonimine with a copper catalyst under mild conditions leads to substituted sulfonamides. Although experimental conditions and workup have not been optimized, best yields have been obtained when using benzyliczinc halides .

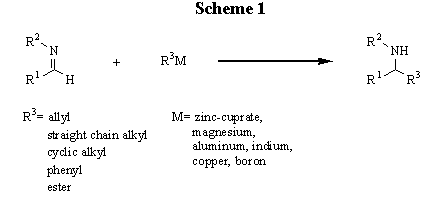
The addition of organometallics to imines is a potentially valuable method for the preparation of secondary amines (scheme 1). However, these types of reactions have typically been plagued by competitive enolization, reduction, and bimolecular coupling reactions caused by the relatively poor electrophilicity of the imine carbon and the competing loss of an a-proton. Due to the attractiveness of organometallic compounds several different methods have been employed in an attempt to circumvent these problems. A large portion of these efforts has focused on allylation using a variety of allyl-metal reagents [1] with only a few processes dealing with direct alkylation of the imine to yield a secondary amine. Reformatsky reagents [2], Grignard [3] reagents, higher order cuprates [4], zinc-copper reagents [4], and dialkyl zincs [5] have all been utilized with some success. However, Reformatsky reagents are limited to a-bromoesters and use of Grignard reagents requires 1-(trimethylsilyl) benzotrialzole and precludes the presence of a significant number of functional groups. Similarly, higher order cuprates limit available functionality due to the method of preparation. Finally, dialkyl-zincs require both an activated imine and essentially stoichiometric amounts of an amino-alcohol reagent.
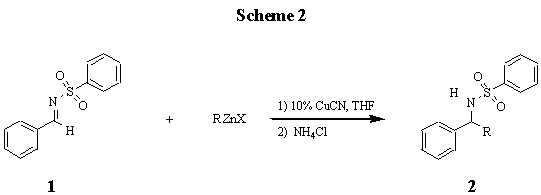
We wish to report here our study on the copper mediated addition of non-allylic organozinc halides to N-benzylbenzensulfonimine in THF (scheme 2). The reaction proceeds smoothly using a catalytic amount of copper cyanide and gives the corresponding sulfonamide product in moderate to excellent yields (table1). Sulfonimines provide an attractive target substrate that seems to have received only small attention [1d]. Sulfonimines are more electrophilic than aldimines and more stable to hydrolysis in aqueous work up. The product sulfonamides are also useful for elaboration of the nitrogen [6] and have been extensively studied with respect to desufonylation to yield amines.[7]
When N-benzyl benzenesulfonimine 1 derived from benzaldehyde and benzensulfonamide, was treated with 4-methoxy benzylzinc chloride [8] (2 mol equiv) in the presence of a sub-stoichiometric amount of copper cyanide (0.2 mol equiv), the corresponding sulfonamide was obtained in 80% isolated yield [9]. Alkylzinc halides generally seem to give disappointing yields (entries 1, 3, 4, and 7); however, it should be noted that none of these reactions have been optimized for highest yield. Even without optimization, benzyliczinc halides containing electron donating groups give generally good to excellent yields (entries 5, 11, and 12). All products were characterized using 1H and 13C NMR, melting point determination, and in some cases C and H elemental analysis
Currently efforts are being focused on optimizing isolated yields via modification of reaction conditions and work up. A secondary effort has been directed towards removal of the sulfonyl group to yield an amine. With the popularity of sulfonyl as an amine protecting group there are several methods currently available to explore.
Table 1
Entry
Organo-zinc
Yield
(%)a
MP(oC)c |
|
a
Isolated yields. b Based on recovered starting material. c Melting points are uncorrected. dMelting points obtained from unrecrystallized productReferences and Endnotes
[1] Representative (but by no means exhaustive) sampling of these types of reactions using zinc, magnesium, boron, higher order cuprates, and indium may be found (a) Basile ,T.; Bocoum, A.; Savoia, D.; Umani-Ronchi A. J. Org. Chem. 1994, 59, 7766. (b) Yamamoto, Y.; Nishii, S.; Maruyama, K.; Komatsu, T.; Ito, W. J. Am. Chem. Soc. 1986, 108, 7778. (c) Boucoum, A.; Bogo, C.; Savoia, D.; Umani-Ronchi, A. Tetrahedron Lett. 1991, 32, 1367. (d) Chan, T.H.; Lu, W. Tetrahedron Lett. 1998, 39, 8605.
[2] For a review of the Reformatsky reaction see: Furstner, A. Synthesis 1989, 571
[3] Katritzky, A.R.; Hong, Q.; Yang, Z. J. Org. Chem. 1995, 60, 3405
[4] Bandini, M.; Cozzi, P.G.; Umani-Ronchi, A.; Villa, M. Tetrahedron 1999, 55, 8103
[5] Soai, K.; Hatanaka, T.; Miyazawa, T. J. Chem. Soc. Chem. Commun 1992 1097
[6] Henry, J.R.; Marcin, L.R.; McIntosh, M.C.; Scola, P.M.; Harris Jr, G.D.; Weinreb, S.M. Tetrahedron Lett. 1989, 30, 5709
[7] (a) Nyasse, B.; Grehn, L.; Ragnarsson, U. Chem. Commun. 1997, 1017. (b) Yasuhara, A.; Sakamoto, T. Tetrahedron Lett. 1998, 39, 595. (c) Knowles, H.; Parsons, A.F.; Pettifer, R.M. Synlett 1997, 271
[8] Organo zinc halides were purchased as a .5M solution in THF under argon in sureseal bottles from Aldrich Chemical Company in all cases excepting the substituted pyridine and the ester. The substituted pyridine was not available from Aldrich and was obtained from Rieke Metals Inc (RMI). The ester zinc bromide was synthesized using Rieke� Zinc (RMI) and literature procedures: Hanson, M.V.; Brown, J.D.; Rieke, R.D.; Niu, Q.J. Tetrahedron Lett. 1994, 35, 7205
[9] 3ml of a .2 molar solution of CuCN/2LiBr (.6mmol) was added to 12ml of a .5 molar solution of 4-methoxy benzylzinc chloride (6mmol) in 10 ml THF under argon. The solution was stirred at room temperature for one minute, then .7388g of Sulfonimine 1 (3.01mmols) dissolved in 10 ml THF was added via cannula needle. The mixture was allowed to react for 12 hours, over which time the solution became black and opaque. Then the reaction was quenched by direct addition of 1.44g ammonium chloride and stirred for 15 minutes. The resulting mixture was filtered to yield a light blue-green solution, which was adsorbed directly onto silica gel. .88 grams of desired product (entry 11, 2.4mmols) was obtained via flash chromatography from silica gel, eluting with a solvent gradient 10%, 15%, 20% ethyl acetate in hexanes. Analytical samples for melting point determination and NMR analysis were obtained through recrystallization from ethyl acetate by addition of hexane.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0034 as the message subject of your e-mail.