
Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0038]
Received: 24 July 2000 / Uploaded: 30 July 2000
Introduction
Several non-proteinogenic amino acids containing the pyrrolidine ring
show high affinity towards NMDA (N-methyl-D-aspartic acid) and glutamate receptors. Thus,
they can be employed in order to establish the mechanisms involved in transmission of
nerve impulse. Moreover, they can be used for treatmentment of CNS diseases. Among them,
2-carboxy-3-pyrrolidinacetic acid (CPAA), 1, and the diastereomeric
2-carboxy-4-(2-methoxyphenyl)-3-pyrrolidineacetic acids 2 and 3 are
significant examples. [1-11]

In particular, compound 1 is highly effective towards NMDA receptors, whereas 3 shows activity higher than kainic acid.
Results and discussion
We devised that D-Xylose 9 could be a useful starting material
for a new synthetic approach to these amino acids in the enantiomerically pure form.[12] The synthesis we planned proceeds according the reported
retrosynthetic scheme, through the formation of the chiral pyrrolidin-2-one 5
which, by removal of the carbonyl group carried out according literature methods, can be
eventually converted into the pyrrolidine ring.

A further challenge relies on the concavity of compound 5. In fact, alkylation at
the a-position with respect to the carbonyl results to be
stereoselective, leading to the exo-alkylated derivative.
Thus, starting from di-O-acetyl-D-xylal, 10, the anomeric 2-pentenopyranosides 11 and 12 were prepared by using a modified Ferrier reaction. [13-14]
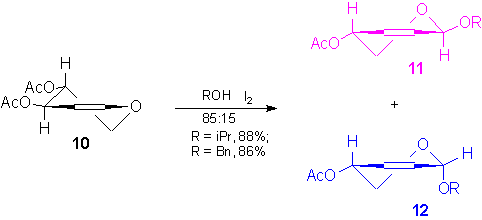
The anomeric mixture was separated by flash chromatography and the
configuration of products was assigned of by 1H NMR. The major anomer, 11,was
then converted into the azidoderivative 14 and subsequently into the corresponding
amine, 15.[15]

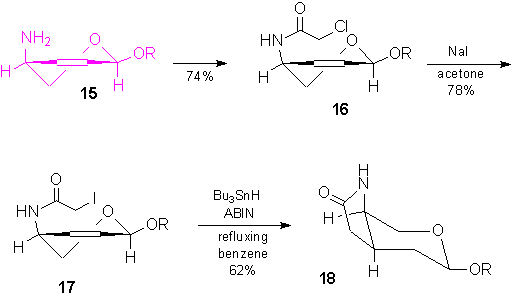
In order to obtain the bicyclic system 18, the amine 15 was acylated with
chloroacetyl chloride and then chloroacetamide 16 was converted into iodoacetamide 17.
Eventually, by treating with Bu3SnH, the bicyclic lactam 18 was isolated
in moderate yield.
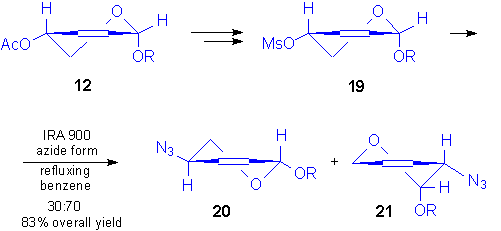
On the contrary, when 4-azido derivative was prepared starting from the minor anomer, 12,
a surprising result was observed. In fact, the corresponding 4-mesyl derivative 19
was prepared, but the nucleophilic substitution, carried out under different conditions
(IRA 900 in the azide form in refluxing benzene, NaN3 in DMF at 80 �C,
diphenylphosphoryl azide) invariably led to two products, the minor component of the
mixture, 20, arising from a SN2 pathway, as determined on the basis of 1H
NMR data.[16] In fact, as it resulted from 1H NMR
spectrum and n.O.e. experiments, the major product of the reaction was the
2-azidoderivative 21, which formed by a SN2� pathway.
With the aim to check the observed effect, the same reaction sequence was carried out starting from benzyl a-2-pentenopyranoside 22, and a mixture of azides 24 and 25 was obtained, in 78% overall yield and 30:70 ratio.

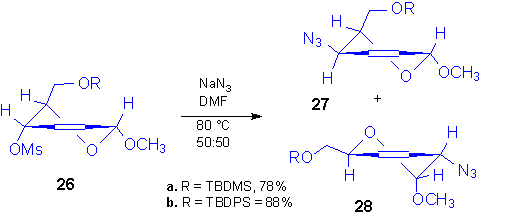
The same effect, which was tentatively ascribed to the configuration of the anomeric carbon, was observed when the same reaction was carried out starting from mesyl derivative 26 prepared starting from methyl a-D-2-hexenopyranosides. In fact, an equimolar mixture of azides 27 and 28 was obtained in good yield, whose configuration was assigned by 1H NMR data, and the substitution process was unaffected even on changing both the reaction conditions and the protecting group at 6-OH.
Allo scopo di spiegare il comportamento dell�anomero a nel corso della reazione di sostituzione del gruppo mesile da parte del gruppo azide, si sono calcolate in via preliminare le energie degli stati di transizione della reazione SN2 e SN2� per gli anomeri a e b.
Le strutture degli stati di transizione sono state localizzate ed ottimizzate a livello RHF/3-21G* e su tali geometrie e stato condotto un calcolo singolo (single point) a livello B3LYP/3-21G* per tener conto della correlazione elettronica. [17-20]
a-SN2 E = -1284.24246521 u.a. i.f.: -299.86 cm-1a-SN2� E = -1284.24706417 u.a. i.f.: -281.88 cm-1
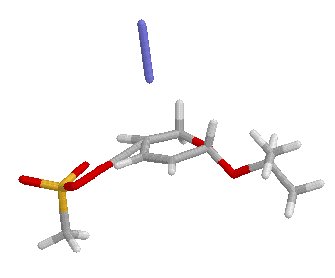
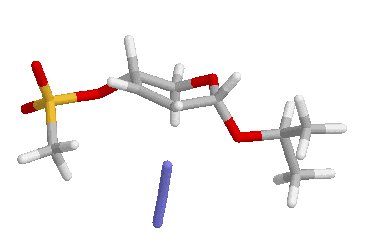
b-SN2 E = -1284.24759963 u.a. i.f.: -323.57 cm-1; b-SN2� E = -1284.2679714 u.a. i.f.: -218.0 cm-1


[1. ] McGeer, G.; Olney, I. W.; McGeer, P. I., Eds. "Kainic acid as a tool
in neurobiology". Raven Press, 1978 , N. Y.
[2.] Shinozaki, H.; Shibuya, I. Neuropharmacology, 1974, 13, 1057.
[3.] Shinozaki, H.; Ishida , M., Brain Research, 1976, 109 , 435 .
[4.] Shinozaki, H.; Shibuya , I. Neuropharmacology, 1974, 13,
665.
[5.] Friedle, N. M.; Kelly, P. H.; Moore, K. E., Brain Pharmacology, 1978, 63,
151.
[6.] Parsons, A.F., Tetrahedron, 1996, 52, 4149.
[7.] Watkins, J.C.; Krogsgaard-Larsen, P.; Honore, T., Trends Pharmacol. Sci., 1990,
11, 25.
[8.] Hashimoto, K.; Horikawa, M.; Shirahama, H., Tetrahedron Lett., 1990, 31,
7047.
[10.] Ishida, M.; Shinozaki, H., Br. J. Pharmacol., 1991, 104, 873.
[11.] Tsai, C.; Schnider, J.A.; Lehmann, J. Neurosci. Lett., 1988, 92,
298.
[12]. Langlois, N.; Andriamialisoa, R.Z., Tetrahedron Lett., 1991, 32,
3057.
[13.] Hanessian, S. �Total synthesis of natural produets: the chirone
approach". Pergamon Press, Oxford, 1983.
[14.] Ferrier, R. I.; Prasad, N., J. Chem. Soc., (C), 1969, 570.
[15.] Koreeda, M.; Houston, T.A.; Shull, B.K.; Klemke, E.; Tuinman, R.J., Synlett, 1995,
90.
[16.] Fraser-Reid B., Bernard J.C., Holder N.L., Yunker M., Can.J.Chem.,1971,49,3038.
[17.] Thompson, A.S.; Humphrey, G.R.; DeMarco, A.M.; Mathre, D.J.; Grabowski, E.J., J.
Org. Chem., 1993, 58, 5886.
[18.] Gaussian 94, M.J.Frish, G.W. Trucks, H.B.Schlegel,
P.M.W. Gill, B.G.Johnson, M.A.Robb, J.R. Cheeseman, T.A.Keth, G.A. Petersson, J.A.
Montgomery, K. Raghavachari, M.A. Al-Laham, V.G. Zakrzewski, J.V. Ortiz, J.B. Foresman, J.
Cioslowski, B.B. Stefanov, A. Nanayakkara, M. Challacombe, C.Y. Peng, P.Y. Ayala, W.Chen,
M.W. Wong, J.L. andres, E.S.Replogle, R. Gomperts, R.L.Martin, D.J.Fox, J.S. Binkley, D.J.
Defrees, J. Baker, J.P.Stewart, M. Head-Gordon, C. Gonzalez, and J.A.Pople, Gaussian,
Inc., Pittsburg PA, 1995.
[19.] C.C. Roothan, Rev.Mod. Phys,. 1951, 23, 69.
[20.] a) C. Lee, W.Yang, and R.G. Parr, Physical Review B, 1988, 37,
785; b) A.D.Becke, Phys.Rev.A, 1988, 38, 3098 ;
c) B. Miehlich, A.Savin, H.Stoll and H.Preuss, Chem.Phys.Lett., 1989, 157,
200;d) A.D.Becke, J.Chem.Phys., 1993, 98, 5648.
[21.] a) H.B. Schlegel, J.Comp.Chem., 3, 214;b) R.Fletcher and M.J.D.Powell,
Comput.J., 6, 163-168,1963;
c) B.A.Murtaugh and R.W.H. Sargent, Comput.J.,1970, 13, 185; d)
J.Baker, J.Comp.Chem., 1986, 7, 385;
e) J.Baker, J.Comp.Chem, 1987, 8, 563; f) H.B. Schlegel, in New
Theoretical Concepts for Understanding Organic Reactions, Ed.J.Bertran, Kluwer
Academic: The Netherlans, 1989, 33-53.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0038 as the message subject of your e-mail.