![]()
Scheme 1
Results
Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0042]
Pilar López-Alvarado, Carmen Avendaño and J. Carlos Menéndez
Departamento de Química Orgánica y Farmacéutica, Facultad de Farmacia.
Universidad Complutense, 28040 Madrid, Spain.
E-mail:[email protected]Received: 22 July 2000 / Uploaded: 29 July 2000
Introduction
A number of b-dicarbonyl
compounds derived from malonic acid, specially malonamides and malonamic
acid esters, have interesting pharmacological properties including antihypertensive
[1], sedative and anticonvulsant [2], antiinflammatory [3], analgesic
[4], M1 selective muscarinic receptor antagonistic [5] activities, among
others. Furthermore, the unique chelating properties of some malonamides
makes them ideal extracting agents for trivalent lanthanides [6],
actinides [7] and other heavy metal ions [8], and has prompted their
use as components of chromatographic stationary phases [9]. b-Amidoesters,
on the other hand, are also common synthetic intermediates in the preparation
of biologically active agents [10] and other interesting organic compounds
[11].
The current methods for the preparation of
these compounds are normally based on the use of Meldrum's acid [12] or
malonic acid monoethyl ester through its conversion into an acyl chloride
[5,13] or via its reaction with DCC or carbonyldiheterocycles [2].
In most cases, these methods give low or moderate yields. We propose here
a new, flexible route to malonamides and malonamic acid esters that exploits
the well-known regioselective reaction of b-ketothioesters
with electrophiles at C-2, and the high reactivity of the thioester group
towards alcohols and amines in the presence of thiophillic metals [14]
(Scheme 1).
![]()
Scheme 1
Results
Our route starts by the preparation of b-amidothioesters
3
by treatment of commercially available tertbutyl acetothioacetate
1
with NaH and an aryl or alkyl isocyanate without isolation of the
intermediate tricarbonyl derivatives 2, which were normally
deacetylated during workup and purification. Compounds 3 were
treated with several amines in the presence of silver trifluoroacetate
[14], yielding diamides 4 in 80-100% yields (Scheme 2).
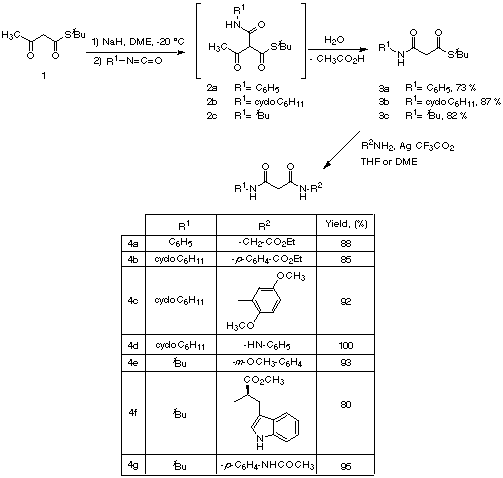
Scheme 2
Similar treatment of compounds 3 with several
alcohols afforded malonamic acid esters 5, also in excellent yields (Scheme
3).

Scheme 3
Finally, we briefly examined the acylation
of bifunctional substrates by compounds 3. Treatment of aminophenol
6
with 2 equivalents of amidoester 3b gave compound 7 in 96%
yield. If only 1 equivalent of the thioester was employed, the reaction
could be made chemoselective, as shown by the preparation of 8 from
6 and 3c (Scheme 4).
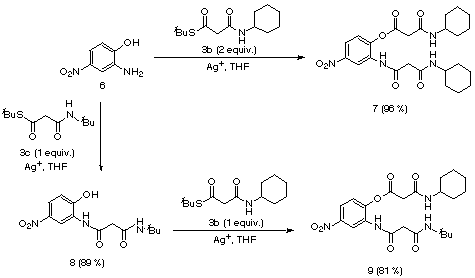
Scheme 4
References
[1] Bernard, R.; Charles, S. J.;
Marie, L. J. Eur. Pat. Appl. EP 38758 [Chem. Abstr.1982,
96,
123304s]
[2] Timothy, T.; David, T. E. Ger.
Offen 2152743 [Chem. Abstr. 1972, 77, 61622r ].
[3] Katagi, T.; Aoki, M.;
Kashiwagi, M.; Ohata, K.; Kohno, S.; Murata, T.; Inoi, T. Chem. Pharm.
Bull. 1985, 33, 4878.
[4] Bernard, R. P.; Evelyne, L.;
Pierre, C.; Jean, C.; Zaluski, F.; and Claude, M. Proc. Natl. Acad.
Sci. USA 1983, 80, 3178.
[5] Turconi, M.; Banfi, A.; Schiavi,
G. B.; Donetti, A. Il Farmaco 1991, 46, 999.
[6] McNamara, B. K.; Lumetta, G.
J.; Rapko, B. M. Solv. Extr. Ion Exch. 1999, 17, 1403.
[7] Iveson, P. B.; Drew, M. G. B.;
Hudson, M. J.; Madic, C. J. Chem. Soc.Dalton Trans. 1999,
3605.
[8] (a) Angus, P. M.; Jackson,
W. G. Inorg. Chim. Acta 1998, 268, 85. (b) Jezierska,
J.; Trochimczuk, A. W.; Kedzierska, J. Polymer 1999, 40,
3611.
[9] Mohapatra, P.; Sriram, S.; Manchanda,
V.; Badheka, L. Separ. Sci. Technol. 1999, 35, 39.
[10] (a) Taylor, E. C.; Liu, B.
Tetrahedron
Lett. , 1999, 40, 5291. (b) Gómez-Parra,
V.; Gómez, M. C.; Sanchez, F.; Stefani, V. Arch.
Pharm., 1992, 325, 483. (c) Galeazzi, R.; Mobbili, G.;
Orena, M. Tetrahedron 1999, 55, 261.
[11] Saalfrank, R. W. ; Lutz,
T.; Hörner, B.; Gündel, J.; Peters, K.; von Schnering, H. G.
Chem. Ber. 1991, 2289.
[12] Gellman, S. H.; Dado, G. P.;
Liang, G. B.; Adams, B. R. J. Am. Chem. Soc. 1991,
113,
1164.
[13] Hamper, B. C.; Kolodziej, S.
A.; Scates, A. M. Tetrahedron Lett. 1998, 39,
2047.
[14] Ley, S. V.; Woodward, P. R. Tetrahedron
Lett.1987, 32, 2431.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0042 as the message subject of your e-mail.