
Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0043]
An Unexpected Result from a 1,3-Dipolar Cycloaddition: Synthesis of Pyrrolo[1,2,3-de]quinoxalines
Raymond C F Jones,*1 James N Iley2 and Pedro M J Lory2
1Chemistry Department, Loughborough University, Loughborough, Leics. LE11
3TU, UK;
E-mail: [email protected]
2
Department of Chemistry, The Open University, Walton Hall, Milton Keynes MK7 6AA, UKReceived: 17 July 2000 / Uploaded: 30 July 2000
Introduction
As part of a programme to evaluate the imidazolinium ylides 2 in 1,3-dipolar cycloaddition reactions [1], we wished to explore intramolecular cycloaddition. The azomethine ylides 2 are prepared (Scheme 1) by alkylation of imidazolines 1 with an active halide such as an a-halo ester (X = CO2R) and addition of a base (DBU); in the presence of a dipolarophile, the dipole 2 undergoes regioselective and diasteroselective cycloaddition (e.g. Scheme 1). Thus in one-pot, three of the five bonds of a new pyrrolidine ring are assembled

Scheme 1
We have utilised this protocol in asymmetric synthesis, starting with optically active 1-benzyl-4-phenyl-2-imidazolines and removing the templating atoms from the new pyrrolidine ring [2]. To render this sequence intramolecular, we determined to employ a haloalkyl reagent carrying the dipolarophile, and have succeeded in doing so with an ester tether (Scheme 2) [3].

Scheme 2
We report herein that attempts to extend this to an all-carbon tether, using an a-haloketone rather than an a-haloester, lead to unexpected post-cycloaddition events, and eventually to the formation of the novel pyrrolo[1,2,3-de]quinoxaline ring system.
Results and Discussion
Thus, when methyl E-8-bromo-7-oxooct-2-enoate 3a was heated with 1-benzyl-2-imidazoline 1 in THF at reflux, and DBU was added dropwise over 4 hours, the expected cycloadduct 4 was not isolated, but instead a product characterised as the pyrrolo[1,2,3-de]quinoxaline 5 was formed (30%) (Scheme 3). The formation of this unexpected novel heterotricycle can be rationalised as shown in Scheme 3, via initial dipolar cycloaddition and eliminative ring-opening of the primary cycloadduct 4 to give the enamino-ester 6; the liberated secondary amine then attacks the ketone carbonyl group [4] to form in the first instance an enamine such as 7, although we cannot be sure of the regiochemistry. In any event, tautomeric shifts of proton then result in formation of the aromatic pyrrole sub-structure and formation of 5. Pyrroloquinoxaline 5 was also the isolated product (20%) when the diastereosomeric dienophile methyl Z-8-bromo-7-oxooct-2-enoate 3b was used in the reaction.
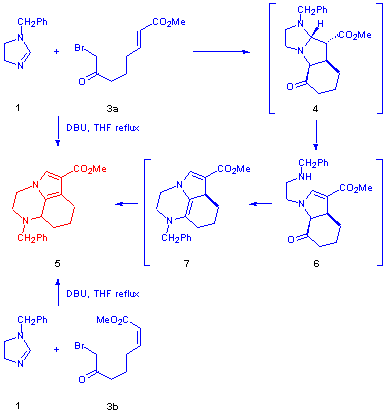
Scheme 3
Other examples of this ring-opening and recyclisation were observed: using 1-benzyl-2-phenyl-2-imidazoline 8 with bromoketone 3a afforded tricycle 9 (32%) (Scheme 4);

Scheme 4
and using 1-benzyl-4-phenyl-2-imidazoline 10 as heterocyclic starting material also with 3a, to give 6-benzyl-1-methoxycarbonyl-4-phenyl-4,5,6a,7-tetrahydro-6H-pyrrolo[1,2,3-de]quinoxaline 11 (30%) (Scheme 5).

Scheme 5
Tricycle 11 was isolated as a crystalline solid that was subjected to X-ray crystallographic examination [5]. This confirmed the structure of the ring system (Figure 1) with the bridgehead hydrogen atom and the 4-phenyl substituent on the same face of the molecule. We are presently exploring the scope of this cycloaddition-rearrangement.
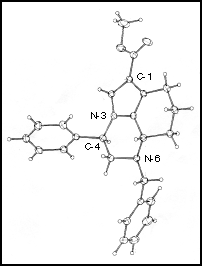

Figure 1: X-Ray crystal structure of 6-benzyl-1-methoxycarbonyl-4-phenyl-4,5,6a,7-tetrahydro-6H-pyrrolo[1,2,3-de]quinoxaline 11
Acknowledgements
We wish to thank the Open University for a studentship (P. M. J. L.), the EPSRC National Crystallography Service Centre (Southampton) for the crystallographic determination, and the EPSRC National Mass Spectrometry Service Centre (Swansea) for some MS data.
References
[1] Jones, R. C. F.; Howard, K. J.; Nichols, J. R.; Snaith, J. S. J. Chem. Soc., Perkin Trans. 1 1998, 2061-2072, and references therein.
[2] Jones, R. C. F.; Howard, K. J.; Snaith, J. S. Tetrahedron Lett. 1996, 37, 1707-1710; Jones, R. C. F.; Howard, K. J.; Snaith, J. S. Tetrahedron Lett. 1996, 37, 1711-1714.
[3] Jones, R. C. F.; Howard, K. J.; Snaith, J. S. Tetrahedron Lett. 1997, 38, 1647-1650.
[4] Cf. Reference 1 for a related recyclization to afford a lactam.
[5] We thank Dr Simon C Coles for this determination.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0043 as the message subject of your e-mail.