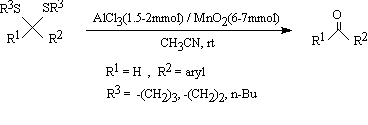
Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0044]
Selective Deprotection of Thioacetals by MnO2, BaMnO4 and KMnO4 in the Presence of Anhydrous AlCl3 and FeCl3 in Dry CH3CN
Habib Firouzabadi*, Hassan Hazarkhani, Babak Karimi, Uranous Niroumand and Soheila Ghassamipour
Department of Chemistry, College of Sciences, Shiraz
University, Shiraz 71454, Iran
Fax : +98(071)20027, E-mail: [email protected]
Received: 8 July 2000 / Uploaded: 29 July 2000
Abstract: Dried manganese dioxide (MnO2), potassium permanganate (KMnO4) and barium manganate (BaMnO4) in the presence of anhydrous aluminum and ferric chlorides performed efficient deprotection of S,S- acetals , 1,3-dithiolanes and 1,3-dithianes, in dry CH3CN at room temperature. Thioacetals derived from enolizable carbonyl compounds remained almost intact.
INTRODUCTION
Protection of carbonyl groups as their open chain and cyclic thioacetals is an important task in the synthesis of organic molecules1. Thioacetals are stable towards ordinary acidic and basic conditions and can act as acyl synthetic equivalent groups. Many procedures are available in the literature for this purpose and along this line, we have recently introduced new efficient methods for this synthetically important transformation2-5.
Deprotection of thioacetals to their carbonyl compounds is not an easy process and straightforward6. Therefore, there is a great demand, in this area of chemistry, for the introduction of mild, efficient, and selective practical methods. Clay supported ammonium7, ferric or cupric nitrates8, zirconium sulfonyl phosphonate9 , oxides of nitrogen10, air / bismuth (III) nitrate11, Fe(phen)3(PF6)312, DDQ13, , SeO2 / AcOH14, hv / pyrylium / O215, N-fluoro-2,4,6-trimethylpyridinium triflate-water system16, methylene green/visible hn 17, SbCl518 / N2, GaCl3 / H2O or GaCl3 / MeOH / O219, , (CF3CO2)2Iph20, m-ClC6H4CO3H / CF3CO2H21, , NaNO3 / aqueous solution (NO+, H2ONO+, ClNO) or t-butyl hypochlorite (Cl+) in anhydrous CCl422, t-butyl bromide (iodide) / DMSO23, DMSO / HCl / H2O24, TMSI (Br) / DMSO25, LiN(i-C3H7)2 / THF26, HgO / 35% aqueous HBF427, benzeneseleninic anhydride28, periodic acid29, , isoamyl nitrite30, O-mesitylenesulfonyl hydroxylamine31, , Et3O+BF4-32, MeI in moist acetone33 or in 96% methanol34, cerric ammonium nitrate (CAN) in aqueous CH3CN35, HgO-BF3 / H2O-THF6, N-chloro-p-toluenesulphonamide (chloroamine-T)36, 1-chlorobenzotriazole37, N-halosuccinimide38, and CuCl2 / CuO39 and hyprervalent (tert-butylperoxy) iodane40/ CH3CN-H2O has been documented in the last two decades.
In spite of extensive studies on the chemistry of MnO2 and KMnO441 and the less studied BaMnO442, a strong competitor of MnO2, dethioacetalization of S, S-acetals, 1,3-dithiolanes and 1,3-dithianes has not been studied by these reagents yet. In this report we have presented new efficient and non-hydrolytic methods for the deprotection of thioacetals derived from aldehydes and non-enolizable ketones using dry MnO2, BaMnO4 and KMnO4 as nucleophiles in the presence of unhydrous AlCl3 and FeCl3 in dry CH3CN. A mechanism has also been proposed for the reactions.
RESULTS AND DISCUSSION
Recently we have found that a mixture of MnO2 and AlCl3 is an effective reagent for selective oxidative deprotection of benzylic trimethylsilyl- and tert-butyldimethylsilyl ethers43. Also we have shown that various types of trimethylsilyl ethers and tert-butyldimethylsilyl ethers deprotected and oxidized by potassium permanganate (KMnO4) and barium manganate (BaMnO4) in the presence of Lewis acids in dry non-aqueous media44. In continuation of our studies for dethioacetalization of acyclic and cyclic dithioacetals in non-aqueous media, we decided to study the reactions of these manganese-based oxidants for this purpose. First we started to explore the behavior of MnO2 in the presence of different hydrated and unhydrous metal salts such as ZnCl2, MnCl2.4H2O, CdCl2.2H2O, ZrOCl2.8H2O, FeCl3, AlCl3, PbCl2, CuSO4.5H2O in aprotic organic solvents for the deprotection of 2-(4-Me-Phenyl)-1,3-dithiane as a model compound. Our observations showed that only anhydrous AlCl3 and FeCl3 were effective catalysts for the promotion of this reaction in dry CH3CN. Addition of AlCl3 or FeCl3 in dry CH3CN in the absence of MnO2 did not effect any changes upon dethioacetalization of thioacetals after two days. Manganese dioxide was also ineffective reagent for this purpose in the absence of AlCl3 and FeCl3. We have also found that the order of the addition of MnO2 and the Lewis acids to the reaction mixture was very crucial. Lewis acids (1.5-2mmol) should be added first to the reaction mixture and the mixture should be stirred for a few minutes before the addition of MnO2 (6-7mmol) to the reaction mixture. The reactions proceeded smoothly and the desired carbonyl compounds were isolated in 82-96% yields (Scheme 1,Table 1).
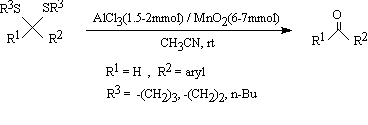
Scheme 1.
Table 1: Deprotection of thioacetals by MnO2 , KMnO4 and BaMnO4 catalyzed with AlCl3 in CH3CN at room temperature
Entry Substrate Time(min) ratio of oxidantsa Yield%
MnO2, KMnO4, BaMnO4 MnO2,KMnO4,BaMnO4 MnO2, KMnO4, BaMnO4
1  90 , 45 , 60
6 , 3 , 4
95 , 94 , 93
90 , 45 , 60
6 , 3 , 4
95 , 94 , 93
2  90 , 50 , 60
6 , 3 , 4
96 , 95 , 95
90 , 50 , 60
6 , 3 , 4
96 , 95 , 95
3 ![]() 100, 60 , 70
7 , 3 , 4
89 , 91 , 90
100, 60 , 70
7 , 3 , 4
89 , 91 , 90
4 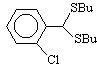 150, 100 , 120
7 , 4 , 5
88 , 90 , 91
150, 100 , 120
7 , 4 , 5
88 , 90 , 91
5  60 , 30 , 45
6 , 3 , 4
93 , 95 , 92
60 , 30 , 45
6 , 3 , 4
93 , 95 , 92
6 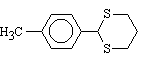 60 , 30 , 40
6 , 3 , 4
92 , 96 , 95
60 , 30 , 40
6 , 3 , 4
92 , 96 , 95
7  60 , 45 ,
45
6 , 3 , 4
90 , 92 , 91
60 , 45 ,
45
6 , 3 , 4
90 , 92 , 91
8  120, 95 , 100
7 , 4 , 5
92 , 90 , 91
120, 95 , 100
7 , 4 , 5
92 , 90 , 91
9 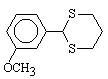 60 , 45 , 55
6 , 3 , 4
91 , 91 , 90
60 , 45 , 55
6 , 3 , 4
91 , 91 , 90
 50 , 40 , 50
6 , 3 , 4
90 , 92 , 90
50 , 40 , 50
6 , 3 , 4
90 , 92 , 90
11  100, 80 ,
95
7 , 4 , 5
91 , 90 , 92
100, 80 ,
95
7 , 4 , 5
91 , 90 , 92
12 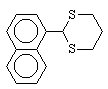 70 , 35 , 50
6 , 3 , 4
92 , 94 , 95
70 , 35 , 50
6 , 3 , 4
92 , 94 , 95
13  70 , 45 , 65
6 , 3 , 4
85 , 84 , 83
70 , 45 , 65
6 , 3 , 4
85 , 84 , 83
14 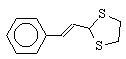 75 , 45 , 60
6 , 3 , 4
82 , 84 , 85
75 , 45 , 60
6 , 3 , 4
82 , 84 , 85
 75 , 50 , 60
6 , 3 , 4
91 , 92 , 90
75 , 50 , 60
6 , 3 , 4
91 , 92 , 90
By this method only benzylic dithioacetals of aldehydes and non-enolizable ketones were converted to their carbonyl compounds. Dithioacetals derived from enolizable ketones were isolated intact from the reaction mixtures (Scheme2, Table 2).
 |
NR |
Scheme 2.
Table 2: The results of deprotection of thioacetals of enolizable ketones by MnO2 , KMnO4 and BaMnO4 in CH3CN at room temperature in the presence of AlCl3
Entry Substrate Time(min) ratio of oxidantsa Yield%
MnO2, KMnO4, BaMnO4 MnO2,KMnO4,BaMnO4 MnO2, KMnO4, BaMnO4
1  120, 120 , 120
7 , 4 , 5
NR , NR, NR
120, 120 , 120
7 , 4 , 5
NR , NR, NR
2  120, 120 , 120
7 , 4 , 5
NR , NR, NR
120, 120 , 120
7 , 4 , 5
NR , NR, NR
 120, 120 , 120
7 , 4 , 5
NR , NR, NR
120, 120 , 120
7 , 4 , 5
NR , NR, NR
4 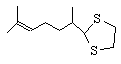 120 , 120 , 120
7 , 4 , 5
NR , NR, NR
120 , 120 , 120
7 , 4 , 5
NR , NR, NR
5 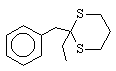 120 , 120 , 120
7 , 4 , 5
NR , NR, NR
120 , 120 , 120
7 , 4 , 5
NR , NR, NR
In order to explain this observation, we have proposed the possibility of formation of a complex between dithioacetals and the Lewis acids in the reaction mixture. These complex does not carry a carbonyl group anymore and therefore, the reaction dose not proceed further (Scheme 3).
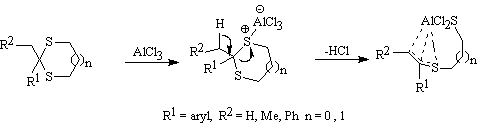
Scheme 3.
In order to avoid a question about the presence of a trace of water in MnO2 powder, that may act as a nucleophile for the cleavage of C-S bonds, unhydrous MnO2 was prepared45 by the decomposition of manganese (II) carbonate (MnCO3) at 220-280� C and AlCl3 sublimed freshly for this purpose. Non-aqueous reaction conditions suggest a non-solvolytic reaction occur in which oxygen of MnO2 acts as a nucleophilic species in this reaction. A mechanism is suggested in which the role of MnO2, as a nucleophile, has been clarified (Scheme 4). A similar conclusion and mechanism has also been proposed for deprotection of S,S-thioacetals by Barton and Ley using seleninic anhydride28.
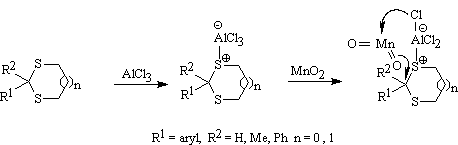

Scheme 4.
Mechanism
These observations prompted us also to study dethioacetalization of S, S- acetals, 1,3- dithiolanes, and 1,3-dithianes by KMnO4, and BaMnO4 in the presence of unhydrous AlCl3 and FeCl3 in dry CH3CN. Both reagents were quite inactive in the absence of the Lewis acids for this purpose. The color of KMnO4 was changed from purple to dark brown but the formation of the carbonyl compounds was not observed at all. The formation of sulfoxides or sulfones was the most probable products formed in the reaction mixture in the absence of Lewis acids. When substituted groups on the aromatic rings bearing a lone-pair of electrons a complex formation between the Lewis acid and the substrates is very probable.This demands a higher molar ratios of the Lewis acids to be used for the reaction to proceed to completion. Our studies show that the rate of the reactions follows the sequence; KMnO4> BaMnO4>MnO2 (Table1). Over-oxidation of the products has not been observed in reactions we have studied. In order to show the usefulness of the procedure, we have compared the results obtained with our presented method with some of those reported in the literature (Scheme5, Table 3).
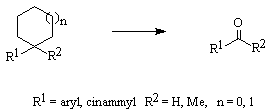
Scheme 5.
Table 3: Comparison of the reported methods with AlCl3 / MnO2 method
Methods
Yields%(min)
R1 R2 n
Ia II18 III13 IV14 V9 VI24
Ph H 1 96(90) _ _ 96(50) 95(60) _
Ph H 2 95(90) _ 70(60) _ 95(60) 97(5)
Ph Me 2 _ _ 75(30) 98(25) 95(30) _
Ph Ph 2 91(75) _ 82(120) _ _ _
4-Me-C6H4 H 2 92(60) 90(10) 88(180) _ _ _
4-Cl-C6H4 H 2 92(120) _ 87(120) _ _ _
Cinammyl H 2 85(70) 86(10) _ _ _ _
4-MeO-C6H4 H 2 90(50) 76(10) 97(180) _ _ _
a ) AlCl3 / MnO2 in CH3CN.
Conclusion
In this study, we have introduced new applications of KMnO4, BaMnO4 and MnO2 as effective reagents for non-hydrolytic deprotection of non-enolizable benzylic thioaectals in the presence of AlCl3. Reactions were proceeded with lower equivalent amounts of KMnO4, BaMnO4 at room temperature in CH3CN in comparison with MnO2 (Scheme 3, Table 2). The presence of Lewis acids in the reaction mixtures was essential and the sequence of the addition of the Lewis acids was also important. Work-up of the reaction mixture was easy and not time-consuming and the yields of the products were excellent.
Acknowledgements
The authors are grateful to The National Research Council of I.R. Iran for grant no.464 and The Shiraz University Research Council for the support of this work.
References
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0044 as the message subject of your e-mail.