Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0047]
Bicyclic Organophosphorus Fluoridates as
Inhibitors of Acetylcholinesterase
S. Furegati, F. Gorla and P. Rüedi*
Institute of Organic Chemistry, University of Zürich,
Winterthurerstrasse 190, CH-8057 Zürich, Switzerland
E-mail: [email protected]
, [email protected]
Received: 29
July 2000 / Uploaded: 31 July 2000
Introduction
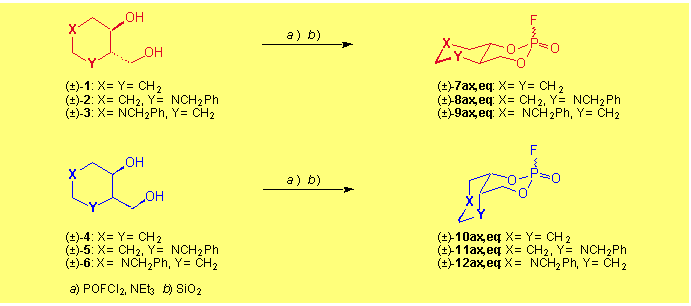
Continuing our investigations (poster1, 2) concerning the irreversible inhibition of serine-hydrolases (acetylcholinesterase, chymotrypsin) by organophosphates [1][2] we have prepared the racemic 3-fluoro-2,4-dioxa-3l5-phosphabicyclo[4.4.0]decan-3-ones (�)-7-(�)-12. Being conformationally restricted, these cis- and trans-decaline-type congeners with the F-substituent in the axial and equatorial position fit differently into the active site of acetylcholinesterase (AChE) as represented by their kass-values.
Synthesis
The bicyclic organophosphates were synthesized from the corresponding diols ((�)-1-(�)-6) with POFCl2 in the presence of triethylamine. Chromatographic separation yielded the axially and equatorially F-substituted epimers. The starting diols have been prepared by reduction of the corresponding oxo-esters: (�)-1/(�)-4 from ethyl 2-oxocyclohexanecarboxylate [2], (�)-3/(�)-6 from ethyl (1-benzyl-3-oxopiperidin-4-yl)carboxylate [3] and chromatographic separation of the cis- and trans-isomers. The piperidine diols (�)-2/(�)-5 were obtained from 3-hydroxy-2-(hydroxymethyl)pyridine after several reaction steps [4].
The X-ray structures of (�)-8ax,eq/(�)-11ax,eq (see the separate Poster A0048) show the influence of the anomeric effect on the equatorially substituted congeners, where the F-substituent adopts a pseudo-axial position.

Inhibitory activity
Irreversible organophosphorous inhibitors form a covalent bond between the activated Ser200 of AChE and the P-atom, F being the leaving group [5]. The resulting tetrahedral phosphate is regarded as a stable transition state analogue of AChE and its natural substrate acetylcholine (ACh) [6].
The kass-values of the irreversible inhibitors were measured according to the method of Baici [7] in the presence of substrate. Some of the compounds seem not to bind covalently to AChE but they interact weekly in a reversible manner. The corresponding KI-values were determined by Lineweaver-Burk plots [8].
As the organophosphates represent N-benzylated, uncharged ACh-mimetics we tentatively conclude that the strongest irreversible inhibitor (�)-8ax is closest to the conformation of ACh during the inhibition.
k ass [M-1min-1]K I [mM] |
axial
|
equatorial |
axial |
equatorial |
|
(�)-7 |
k ass = 1100 |
k ass = 350 |
(�)-10 |
K I = 225 |
k ass = 490 |
(�)-8 |
k ass = 2100 |
k ass = 360 |
(�)-11 |
k ass = 325 |
K I = 1000 |
(�)-9 |
k ass = 570 |
k ass = 1200 |
(�)-12 |
k ass = 470 |
K I= 500 |
[1] F.A. Merckling, P. Rüedi, Chimia 1994, 48, 279; F.A. Merckling, P. Rüedi, Tetrahedron Letters 1996, 37, 2217.
[2] W. Ganci, E.J.M. Meier, F.A. Merckling, G. Przibille, U. Ringeisen, P. Rüedi, Helv. Chim. Acta, 1997, 80, 421;
S. Furegati, W. Ganci, G. Przibille, P. Rüedi, Helv. Chim. Acta, 1998, 81, 1127.
[3] U. Ringeisen, Ph.D. Thesis, University of Zurich, 1996.
[4] S. Furegati, Ph.D. Thesis, University of Zurich, in preparation.
[5] D.M. Quinn, Chem.Rev. 1987, 87, 955; J.L. Sussman, I. Silman, Curr. Opin. Struct. Biol. 1992, 2, 271.
[6] R. Wolfenden, Acc. Chem. Res. 1972, 5, 10; G.E. Lienhard, Science 1973, 180, 149.
[7] H. Früh, G. Kostoulas, B.A. Michel, A. BAICI, Biol. Chem. 1996, 377, 579; A. BAICI, Biol. Chem. 1998, 379, 1007.
[8] E.A. Dawes, 'Quantitative Problems in Biochemistry', Longman, London and New York, 1980.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0047 as the message subject of your e-mail.