
Fourth International Electronic Conference on Synthetic Organic Chemistry (ECSOC-4), www.mdpi.org/ecsoc-4.htm, September 1-30, 2000
[A0048]
X-Ray Structures of Bicyclic Organophosphates
Illustrating the Anomeric Effect
S. Furegati, A. Linden and P. Rüedi*
Institute of Organic Chemistry, University of Zürich,
Winterthurerstrasse 190, CH-8057 Zürich, Switzerland
E-mail: [email protected]
, [email protected]
Received: 21
July 2000 / Uploaded: 30 July 2000
Introduction
The influence of the anomeric effect on bicyclic organophosphates could be shown for the first time by means of X-ray analysis.
In the course of our syntheses of bicyclic organophosphates as inhibitors of acetylcholinesterase we have been able to crystallize a series of four isomeric compounds: (�)-7-aza-7-benzyl-3-fluoro-2,4-dioxa-3l5-phosphabicyclo[4.4.0]decan-3-ones (�)-1ax,eq and (�)-2ax,eq, cis- and trans-decaline-type congeners with the F-substituent in the axial and equatorial position.
The syntheses of the mentioned and of some related compounds as well as their inhibitory effects on acetylcholinesterase are described on a separate Poster A0047.

X-Ray Structures
(�)-1ax,eq and (�)-2ax,eq had been crystallized and submitted to X-ray analysis. As expected, both axially substituted isomers adopt the sterically and stereoelectronically favoured double-chair conformation in the crystals (as well as in solution).
For the two equatorially substituted compounds the electronegative substituent (F) tends to occupy the sterically unfavoured pseudo-axial position according to the anomeric effect [1]. This results e.g. in a chair-twistboat conformation. Although we showed by means of NMR-spectroscopy that in solution (�)-1eq and (�)-2eq exist in a mixture of double-chair and chair-twistboat conformations ((�)-1eq in CD2Cl2: 33:67 at 293 K, 21:79 at 213 K) we obtained crystals of a chair-twistboat form ((�)-1eq) and a chair-envelope form ((�)-2eq) as they seem to have a slightly lower DG than the double-chair forms. The envelope conformation is highly surprising, it represents a middle position between the two possible twistboat conformations with five ring-atoms in plane, only C(6) laying outside.
This is the first time the anomeric effect could be illustrated by X-ray analysis of such bicylic phosphates. Former attempts to crystallize related compounds lead to X-ray structures only with double-chair conformations, the electronegative substituent (O-phenyl, O-4-methoxyphenyl, N(CH2CH2Cl)2, resp.) being located in the axial resp. equatorial position [2], see also poster. However, a 1,4-bis-t-butyl substituted monocylic organophosphate yielded an X-ray structure showing a boat conformation [3].

(�)-1ax (double-chair)
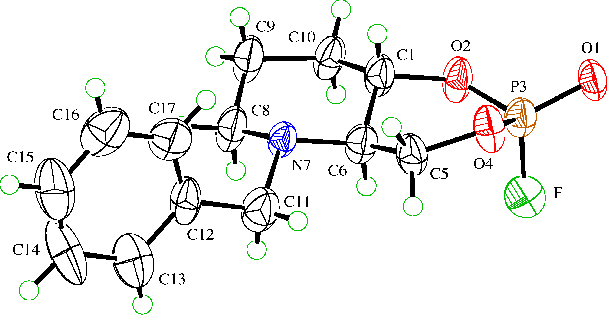
(�)-1eq (chair-twistboat)

(�)-2ax (double-chair)

(�)-2eq (chair-envelope)
Conformation analysis in solution (CDCl3) was carried out by means of 31P- and 1H-NMR-spectroscopy using the 3JPH-coupling constants. According to the Karplus-equation a torsion angle between P�O�C�H of 180� leads to a maximal 3JPH ca. 25 Hz, while a 90� angle corresponds with zero. The characteristic pattern of the three 3JPH-coupling constants in each compound gives information about the conformations ocurring in solution [4].
References[1] D.G. Gorenstein, R. Rowell, J. Findlay, J. Am. Chem. Soc. 1979, 102, 5077.
[2] R.O. Day, D.G. Gorenstein, R.R. Holmes, Inorg. Chem. 1983, 22, 2192; P. Van Nuffel, A.T.H. Lenstra, H.J. Geise,
Bull. Soc. Chim. Belg. 1982, 91; J.-C. Yang, D.O. Shah, N.U.M. Rao, W.A. Freeman, G. Sosnovsky, D.G. Gorenstein,
Tetrahedron 1988, 44, 6305.
[3] R.O. Day, W.G. Bentrude, K.C. Yee, W.N. Setzer, J.A. Deiters, R.R. Holmes, J. Am. Chem. Soc. 1984, 106, 103.
[4] S. Furegati, PhD Thesis, University of Zurich, in preparation.
All comments on this poster should be sent by e-mail to (mailto:[email protected] ona.edu)
[email protected] with A0048 as the message subject of your e-mail.